Ultrastructural Evidence of Synapse Preservation and Axonal Regeneration Following Spinal Root Repair with Fibrin Biopolymer and Therapy with Dimethyl Fumarate
Abstract
:1. Introduction
2. Materials and Methods
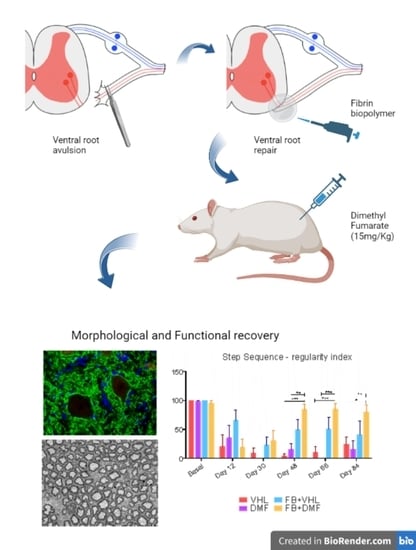
2.1. Experimental Animals and Groups
2.2. VRA and Lesioned Motor Root Reimplantation
2.3. Specimen Preparation
2.4. Immunofluorescence
2.5. Sciatic Nerve Regeneration
2.6. Synaptic Analysis of the Ultrathin Sections
2.7. Motor Function Recovery Assessment
2.8. Statistical Analysis
3. Results
3.1. Synapse Preservation after Ventral Root Avulsion and DMF Treatment
3.2. Ultrastructural Analysis of Alpha Motoneurons
3.3. Nerve Terminals Distribution
3.4. Neurons with Incomplete Regeneration
3.5. Nerve Preservation
3.6. Sciatic Nerve Histomorphometry
3.6.1. Myelinated Axon Diameter (FD)
3.6.2. Axon Diameter (AD)
3.6.3. Myelin Sheath Thickness (MST)
3.6.4. ’g’ Ratio
3.7. Motor Functional Recovery
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Bonney, G.; Birch, R.; Jamieson, A.M.; Eames, R.A. Experience with vascularized nerve grafts. Clin. Plast. Surg. 1984, 11, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Carlstedt, T.P. Spinal nerve root injuries in brachial plexus lesions: Basic science and clinical application of new surgical strategies. A review. Microsurgery 1995, 16, 13–16. [Google Scholar] [CrossRef] [PubMed]
- Carlstedt, T. Experimental studies on surgical treatment of avulsed spinal nerve roots in brachial plexus injury. J. Hand Surg. Br. 1991, 16, 477–482. [Google Scholar] [CrossRef]
- Carlstedt, T.; Cullheim, S.; Risling, M.; Ulfhake, B. Nerve fibre regeneration across the PNS-CNS interface at the root-spinal cord junction. Brain Res. Bull. 1989, 22, 93–102. [Google Scholar] [CrossRef]
- Carlstedt, T.; James, N.; Risling, M. Surgical reconstruction of spinal cord circuit provides functional return in humans. Neural Regen. Res. 2017, 12, 1960–1963. [Google Scholar] [CrossRef] [PubMed]
- Carlstedt, T.; Linda, H.; Cullheim, S.; Risling, M. Reinnervation of hind limb muscles after ventral root avulsion and implantation in the lumbar spinal cord of the adult rat. Acta Physiol. Scand. 1986, 128, 645–646. [Google Scholar] [CrossRef]
- Carlstedt, T.; Risling, M.; Linda, H.; Cullheim, S.; Ulfhake, B.; Sjogren, A.M. Regeneration after spinal nerve root injury. Restor. Neurol. Neurosci. 1990, 1, 289–295. [Google Scholar] [CrossRef]
- Cullheim, S.; Carlstedt, T.; Hallin, R.; Linda, H.; Risling, M. Motor neurons regenerate axons after CNS-injury. Surgery in cases of rotavulsion restore motor functions. Lakartidningen 1996, 93, 4537–4541. [Google Scholar]
- Cullheim, S.; Carlstedt, T.; Linda, H.; Risling, M.; Ulfhake, B. Motoneurons reinnervate skeletal muscle after ventral root implantation into the spinal cord of the cat. Neuroscience 1989, 29, 725–733. [Google Scholar] [CrossRef]
- Cullheim, S.; Risling, M. Observations on the morphology and the axon conduction velocity of axotomized and regenerating sciatic motoneurons in the kitten. Exp. Brain Res. 1982, 45, 428–432. [Google Scholar] [CrossRef]
- Cullheim, S.; Wallquist, W.; Hammarberg, H.; Linda, H.; Piehl, F.; Carlstedt, T.; Risling, M. Properties of motoneurons underlying their regenerative capacity after axon lesions in the ventral funiculus or at the surface of the spinal cord. Brain Res. Brain Res. Rev. 2002, 40, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Koliatsos, V.E.; Price, W.L.; Pardo, C.A.; Price, D.L. Ventral root avulsion: An experimental model of death of adult motor neurons. J. Comp. Neurol. 1994, 342, 35–44. [Google Scholar] [CrossRef]
- Cullheim, S.; Carlstedt, T.; Risling, M. Axon regeneration of spinal motoneurons following a lesion at the cord-ventral root interface. Spinal Cord. 1999, 37, 811–819. [Google Scholar] [CrossRef] [Green Version]
- Vidigal de Castro, M.; Barbizan, R.; Seabra Ferreira, R., Jr.; Barraviera, B.; Leite Rodrigues de Oliveira, A. Direct Spinal Ventral Root Repair following Avulsion: Effectiveness of a New Heterologous Fibrin Sealant on Motoneuron Survival and Regeneration. Neural Plast. 2016, 2016, 2932784. [Google Scholar] [CrossRef] [Green Version]
- Chan, S.Y.; Kuo, C.W.; Liao, T.T.; Peng, C.W.; Hsieh, T.H.; Chang, M.Y. Time-course gait pattern analysis in a rat model of foot drop induced by ventral root avulsion injury. Front. Hum. Neurosci. 2022, 16, 972316. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, X.; Huang, Q.; Dou, Y.; Qu, C.; Xu, Q.; Yuan, Q.; Xian, Y.F.; Lin, Z.X. Quercetin enhances survival and axonal regeneration of motoneurons after spinal root avulsion and reimplantation: Experiments in a rat model of brachial plexus avulsion. Inflamm. Regen. 2022, 42, 56. [Google Scholar] [CrossRef]
- Torok, D.G.; Fekecs, Z.; Pajer, K.; Pinter, S.; Nogradi, A. The use of a detailed video-based locomotor pattern analysis system to assess the functional reinnervation of denervated hind limb muscles. J. Neurosci. Methods 2022, 365, 109398. [Google Scholar] [CrossRef]
- Zhong, K.; Huang, Y.; Zilundu, P.L.M.; Wang, Y.; Zhou, Y.; Yu, G.; Fu, R.; Chung, S.K.; Tang, Y.; Cheng, X.; et al. Motor neuron survival is associated with reduced neuroinflammation and increased autophagy after brachial plexus avulsion injury in aldose reductase-deficient mice. J. Neuroinflamm. 2022, 19, 271. [Google Scholar] [CrossRef]
- Eggers, R.; de Winter, F.; Tannemaat, M.R.; Malessy, M.J.A.; Verhaagen, J. GDNF Gene Therapy to Repair the Injured Peripheral Nerve. Front. Bioeng. Biotechnol. 2020, 8, 583184. [Google Scholar] [CrossRef] [PubMed]
- Eggers, R.; de Winter, F.; Smit, L.; Luimens, M.; Muir, E.M.; Bradbury, E.J.; Tannemaat, M.R.; Verhaagen, J. Combining timed GDNF and ChABC gene therapy to promote long-distance regeneration following ventral root avulsion and repair. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2020, 34, 10605–10622. [Google Scholar] [CrossRef] [PubMed]
- Gunther, M.; Skold, M.K. Temporal gene expression changes after acute and delayed ventral root avulsion-reimplantation. Restor. Neurol. Neurosci. 2020, 38, 23–40. [Google Scholar] [CrossRef] [PubMed]
- Kreutzberg, G.W. Acute Neural Reaction to Injury; Springer: Berlin/Heidelberg, Germany, 1982; pp. 57–69. [Google Scholar]
- Linda, H.; Shupliakov, O.; Ornung, G.; Ottersen, O.P.; Storm-Mathisen, J.; Risling, M.; Cullheim, S. Ultrastructural evidence for a preferential elimination of glutamate-immunoreactive synaptic terminals from spinal motoneurons after intramedullary axotomy. J. Comp. Neurol. 2000, 425, 10–23. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.L.; Thams, S.; Lidman, O.; Piehl, F.; Hokfelt, T.; Karre, K.; Linda, H.; Cullheim, S. A role for MHC class I molecules in synaptic plasticity and regeneration of neurons after axotomy. Proc. Natl. Acad. Sci. USA 2004, 101, 17843–17848. [Google Scholar] [CrossRef] [PubMed]
- Carlstedt, T.; Cullheim, S. Spinal cord motoneuron maintenance, injury and repair. Prog. Brain Res. 2000, 127, 501–514. [Google Scholar] [PubMed]
- Takata, M.; Nagahama, T. Synaptic Efficacy of Inhibitory Synapses in Hypoglossal Motoneurons after Transection of the Hypoglossal Nerves. Neuroscience 1983, 10, 23–29. [Google Scholar] [CrossRef]
- Delgado-Garcia, J.M.; Del Pozo, F.; Spencer, R.F.; Baker, R. Behavior of neurons in the abducens nucleus of the alert cat--III. Axotomized motoneurons. Neuroscience 1988, 24, 143–160. [Google Scholar] [CrossRef]
- Rodrigues Hell, R.C.; Silva Costa, M.M.; Goes, A.M.; Oliveira, A.L. Local injection of BDNF producing mesenchymal stem cells increases neuronal survival and synaptic stability following ventral root avulsion. Neurobiol. Dis. 2009, 33, 290–300. [Google Scholar] [CrossRef]
- Kempe, P.R.G.; Chiarotto, G.B.; Barraviera, B.; Ferreira, R.S., Jr.; de Oliveira, A.L.R. Neuroprotection and immunomodulation by dimethyl fumarate and a heterologous fibrin biopolymer after ventral root avulsion and reimplantation. J. Venom. Anim. Toxins Incl. Trop. Dis. 2020, 26, e20190093. [Google Scholar] [CrossRef]
- Thomazini-Santos, I.A.; Giannini, M.J.S.M.; Toscano, E.; Machado, P.E.A.; Lima, C.R.G.; Barraviera, B. The evaluation of clotting time in bovine thrombin, reptilase®, and thrombin-like fraction of Crotalus durissus terrificus venom using bovine, equine, ovine bubaline and human crioprecipitates. J. Venom. Anim. Toxins 1998, 4, 16. [Google Scholar] [CrossRef]
- Barros, L.C.; Soares, A.M.; Costa, F.L.; Rodrigues, V.M.; Fuly, A.L.; Giglio, J.R.; Gallacci, M.; Thomazini-Santos, I.A.; Barraviera, S.R.C.S.; Barraviera, B.; et al. Biochemical and biological evaluation of gyroxin isolated from Crotalus durissus terrificus venom. J. Venom. Anim. Toxins Incl. Trop. Dis. 2011, 17, 23–33. [Google Scholar] [CrossRef] [Green Version]
- Gasparotto, V.P.; Landim-Alvarenga, F.C.; Oliveira, A.L.; Simoes, G.F.; Lima-Neto, J.F.; Barraviera, B.; Ferreira, R.S., Jr. A new fibrin sealant as a three-dimensional scaffold candidate for mesenchymal stem cells. Stem Cell Res. Ther. 2014, 5, 78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbade, L.P.F.; Barraviera, S.; Silvares, M.R.C.; Lima, A.; Haddad, G.R.; Gatti, M.A.N.; Medolago, N.B.; Rigotto Carneiro, M.T.; Dos Santos, L.D.; Ferreira, R.S., Jr.; et al. Treatment of Chronic Venous Ulcers With Heterologous Fibrin Sealant: A Phase I/II Clinical Trial. Front. Immunol. 2021, 12, 627541. [Google Scholar] [CrossRef] [PubMed]
- Ferreira Junior, R.S.; de Barros, L.C.; Abbade, L.P.F.; Barraviera, S.R.C.S.; Silvares, M.R.C.; de Pontes, L.G.; Dos Santos, L.D.; Barraviera, B. Heterologous fibrin sealant derived from snake venom: From bench to bedside—An overview. J. Venom. Anim. Toxins Incl. Trop. Dis. 2017, 23, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barros, L.C.; Ferreira, R.S., Jr.; Barraviera, S.R.; Stolf, H.O.; Thomazini-Santos, I.A.; Mendes-Giannini, M.J.; Toscano, E.; Barraviera, B. A new fibrin sealant from Crotalus durissus terrificus venom: Applications in medicine. J. Toxicol. Environ. Health. Part. B Crit. Rev. 2009, 12, 553–571. [Google Scholar] [CrossRef]
- Conradi, S. Observations on the ultrastructure of the axon hillock and initial axon segment of lumbosacral motoneurons in the cat. Acta Physiol. Scandinavica. Suppl. 1969, 332, 65–84. [Google Scholar]
- Conradi, S.; Kellerth, J.O.; Berthold, C.H. Electron microscopic studies of serially sectioned cat spinal alpha-motoneurons. II. A method for the description of architecture and synaptology of the cell body and proximal dendritic segments. J. Comp. Neurol. 1979, 184, 741–754. [Google Scholar] [CrossRef]
- Bain, J.R.; Mackinnon, S.E.; Hunter, D.A. Functional evaluation of complete sciatic, peroneal, and posterior tibial nerve lesions in the rat. Plast. Reconstr. Surg. 1989, 83, 129–138. [Google Scholar] [CrossRef]
- Barbizan, R.; Oliveira, A.L. Impact of acute inflammation on spinal motoneuron synaptic plasticity following ventral root avulsion. J. Neuroinflamm. 2010, 7, 29. [Google Scholar] [CrossRef] [Green Version]
- Barbizan, R.; Castro, M.V.; Rodrigues, A.C.; Barraviera, B.; Ferreira, R.S.; Oliveira, A.L. Motor recovery and synaptic preservation after ventral root avulsion and repair with a fibrin sealant derived from snake venom. PLoS ONE 2013, 8, e63260. [Google Scholar] [CrossRef] [Green Version]
- Barbizan, R.; Castro, M.V.; Ferreira, R.S., Jr.; Barraviera, B.; Oliveira, A.L. Long-term spinal ventral root reimplantation, but not bone marrow mononuclear cell treatment, positively influences ultrastructural synapse recovery and motor axonal regrowth. Int. J. Mol. Sci. 2014, 15, 19535–19551. [Google Scholar] [CrossRef] [Green Version]
- Kachramanoglou, C.; Li, D.; Andrews, P.; East, C.; Carlstedt, T.; Raisman, G.; Choi, D. Novel strategies in brachial plexus repair after traumatic avulsion. Br. J. Neurosurg. 2011, 25, 16–27. [Google Scholar] [CrossRef]
- Brannstrom, T.; Kellerth, J.O. Changes in synaptology of adult cat spinal alpha-motoneurons after axotomy. Exp. Brain Res. 1998, 118, 1–13. [Google Scholar] [PubMed]
- Hoang, T.X.; Nieto, J.H.; Dobkin, B.H.; Tillakaratne, N.J.; Havton, L.A. Acute implantation of an avulsed lumbosacral ventral root into the rat conus medullaris promotes neuroprotection and graft reinnervation by autonomic and motor neurons. Neuroscience 2006, 138, 1149–1160. [Google Scholar] [CrossRef]
- Hammarberg, H.; Lidman, O.; Lundberg, C.; Eltayeb, S.Y.; Gielen, A.W.; Muhallab, S.; Svenningsson, A.; Linda, H.; van Der Meide, P.H.; Cullheim, S.; et al. Neuroprotection by encephalomyelitis: Rescue of mechanically injured neurons and neurotrophin production by CNS-infiltrating T and natural killer cells. J. Neurosci. Off. J. Soc. Neurosci. 2000, 20, 5283–5291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carlstedt, T. Functional recovery after ventral root avulsion and implantation in the spinal cord. Clin. Neurol. Neurosurg. 1993, 95, S109–S111. [Google Scholar] [CrossRef] [PubMed]
- Carlstedt, T. Root repair review: Basic science background and clinical outcome. Restor. Neurol. Neurosci. 2008, 26, 225–241. [Google Scholar]
- Carlstedt, T. Nerve root replantation. Neurosurg. Clin. N. Am. 2009, 20, 39–50. [Google Scholar] [CrossRef]
- Purves, D. Functional and structural changes in mammalian sympathetic neurones following interruption of their axons. J. Physiol. 1975, 252, 429–463. [Google Scholar] [CrossRef]
- Kachramanoglou, C.; De Vita, E.; Thomas, D.L.; Wheeler-Kingshott, C.A.; Balteau, E.; Carlstedt, T.; Choi, D.; Thompson, A.J.; Ciccarelli, O. Metabolic changes in the spinal cord after brachial plexus root re-implantation. Neurorehabilit. Neural Repair. 2013, 27, 118–124. [Google Scholar] [CrossRef] [Green Version]
- Oliet, S.H.; Piet, R.; Poulain, D.A. Control of glutamate clearance and synaptic efficacy by glial coverage of neurons. Science 2001, 292, 923–926. [Google Scholar] [CrossRef]
- Wang, D.D.; Bordey, A. The astrocyte odyssey. Prog. Neurobiol. 2008, 86, 342–367. [Google Scholar] [CrossRef]
- Tom, V.J.; Steinmetz, M.P.; Miller, J.H.; Doller, C.M.; Silver, J. Studies on the development and behavior of the dystrophic growth cone, the hallmark of regeneration failure, in an in vitro model of the glial scar and after spinal cord injury. J. Neurosci. Off. J. Soc. Neurosci. 2004, 24, 6531–6539. [Google Scholar] [CrossRef] [Green Version]
- Aldskogius, H.; Liu, L.; Svensson, M. Glial responses to synaptic damage and plasticity. J. Neurosci. Res. 1999, 58, 33–41. [Google Scholar] [CrossRef]
- Lindå, H.; Risling, M.; Cullheim, S. ‘Dendraxons’ in regenerating motoneurons in the cat: Do dendrites generate new axons after central axotomy? Brain Res. 1985, 358, 329–333. [Google Scholar] [CrossRef]
- Parodi, B.; Rossi, S.; Morando, S.; Cordano, C.; Bragoni, A.; Motta, C.; Usai, C.; Wipke, B.T.; Scannevin, R.H.; Mancardi, G.L.; et al. Fumarates modulate microglia activation through a novel HCAR2 signaling pathway and rescue synaptic dysregulation in inflamed CNS. Acta Neuropathol. 2015, 130, 279–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taniuchi, M.; Clark, H.B.; Johnson, E.M., Jr. Induction of nerve growth factor receptor in Schwann cells after axotomy. Proc. Natl. Acad. Sci. USA 1986, 83, 4094–4098. [Google Scholar] [CrossRef] [PubMed]
- Taniuchi, M.; Clark, H.B.; Schweitzer, J.B.; Johnson, E.M., Jr. Expression of nerve growth factor receptors by Schwann cells of axotomized peripheral nerves: Ultrastructural location, suppression by axonal contact, and binding properties. J. Neurosci. Off. J. Soc. Neurosci. 1988, 8, 664–681. [Google Scholar] [CrossRef] [Green Version]
- Lewin, S.L.; Utley, D.S.; Cheng, E.T.; Verity, A.N.; Terris, D.J. Simultaneous treatment with BDNF and CNTF after peripheral nerve transection and repair enhances rate of functional recovery compared with BDNF treatment alone. Laryngoscope 1997, 107, 992–999. [Google Scholar] [CrossRef]
- Zochodne, D.W.; Cheng, C. Neurotrophins and other growth factors in the regenerative milieu of proximal nerve stump tips. J. Anat. 2000, 196 Pt 2, 279–283. [Google Scholar] [CrossRef]
- Tona, A.; Perides, G.; Rahemtulla, F.; Dahl, D. Extracellular matrix in regenerating rat sciatic nerve: A comparative study on the localization of laminin, hyaluronic acid, and chondroitin sulfate proteoglycans, including versican. J. Histochem. Cytochem. Off. J. Histochem. Soc. 1993, 41, 593–599. [Google Scholar] [CrossRef] [Green Version]
- Al-Jaderi, Z.; Maghazachi, A.A. Utilization of Dimethyl Fumarate and Related Molecules for Treatment of Multiple Sclerosis, Cancer, and Other Diseases. Front. Immunol. 2016, 7, 278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albrecht, P.; Bouchachia, I.; Goebels, N.; Henke, N.; Hofstetter, H.H.; Issberner, A.; Kovacs, Z.; Lewerenz, J.; Lisak, D.; Maher, P.; et al. Effects of dimethyl fumarate on neuroprotection and immunomodulation. J. Neuroinflamm. 2012, 9, 163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbizan, R.; Castro, M.V.; Barraviera, B.; Ferreira, R.S., Jr.; Oliveira, A.L. Influence of Delivery Method on Neuroprotection by Bone Marrow Mononuclear Cell Therapy following Ventral Root Reimplantation with Fibrin Sealant. PLoS ONE 2014, 9, e105712. [Google Scholar] [CrossRef]
- Kishino, A.; Ishige, Y.; Tatsuno, T.; Nakayama, C.; Noguchi, H. BDNF prevents and reverses adult rat motor neuron degeneration and induces axonal outgrowth. Exp. Neurol. 1997, 144, 273–286. [Google Scholar] [CrossRef]
- Brock, J.H.; Rosenzweig, E.S.; Blesch, A.; Moseanko, R.; Havton, L.A.; Edgerton, V.R.; Tuszynski, M.H. Local and remote growth factor effects after primate spinal cord injury. J. Neurosci. Off. J. Soc. Neurosci. 2010, 30, 9728–9737. [Google Scholar] [CrossRef] [Green Version]
- Hoffman, P.N.; Griffin, J.W.; Price, D.L. Control of axonal caliber by neurofilament transport. J. Cell Biol. 1984, 99, 705–714. [Google Scholar] [CrossRef]
- Cordaro, M.; Casili, G.; Paterniti, I.; Cuzzocrea, S.; Esposito, E. Fumaric Acid Esters Attenuate Secondary Degeneration after Spinal Cord Injury. J. Neurotrauma 2017, 34, 3027–3040. [Google Scholar] [CrossRef]
- Ikeda, M.; Oka, Y. The relationship between nerve conduction velocity and fiber morphology during peripheral nerve regeneration. Brain Behav. 2012, 2, 382–390. [Google Scholar] [CrossRef]
- Waxman, S.G. Determinants of conduction velocity in myelinated nerve fibers. Muscle Nerve 1980, 3, 141–150. [Google Scholar] [CrossRef]
- Waxman, S.G.; Foster, R.E. Development of the axon membrane during differentiation of myelinated fibres in spinal nerve roots. Proc. R. Soc. London. Ser. B Contain. Pap. A Biol. Character. R. Soc. 1980, 209, 441–446. [Google Scholar]
- Schmalbruch, H. Fiber composition of the rat sciatic nerve. Anat. Rec. 1986, 215, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Han, R.; Xiao, J.; Zhai, H.; Hao, J. Dimethyl fumarate attenuates experimental autoimmune neuritis through the nuclear factor erythroid-derived 2-related factor 2/hemoxygenase-1 pathway by altering the balance of M1/M2 macrophages. J. Neuroinflamm. 2016, 13, 97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, S.X.; Lisi, L.; Dello Russo, C.; Polak, P.E.; Sharp, A.; Weinberg, G.; Kalinin, S.; Feinstein, D.L. The anti-inflammatory effects of dimethyl fumarate in astrocytes involve glutathione and haem oxygenase-1. ASN Neuro 2011, 3, AN20100033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Linker, R.A.; Lee, D.H.; Ryan, S.; van Dam, A.M.; Conrad, R.; Bista, P.; Zeng, W.; Hronowsky, X.; Buko, A.; Chollate, S.; et al. Fumaric acid esters exert neuroprotective effects in neuroinflammation via activation of the Nrf2 antioxidant pathway. Brain A J. Neurol. 2011, 134, 678–692. [Google Scholar] [CrossRef] [Green Version]
- Szepanowski, F.; Donaldson, D.M.; Hartung, H.P.; Mausberg, A.K.; Kleinschnitz, C.; Kieseier, B.C.; Stettner, M. Dimethyl fumarate accelerates peripheral nerve regeneration via activation of the anti-inflammatory and cytoprotective Nrf2/HO-1 signaling pathway. Acta Neuropathol. 2017, 133, 489–491. [Google Scholar] [CrossRef]
- Fraher, J.P. The transitional zone and CNS regeneration. J. Anat. 1999, 194 Pt 2, 161–182. [Google Scholar] [CrossRef]
- Jing, X.; Shi, H.; Zhang, C.; Ren, M.; Han, M.; Wei, X.; Zhang, X.; Lou, H. Dimethyl fumarate attenuates 6-OHDA-induced neurotoxicity in SH-SY5Y cells and in animal model of Parkinson’s disease by enhancing Nrf2 activity. Neuroscience 2015, 286, 131–140. [Google Scholar] [CrossRef]
- Mendell, L.M.; Munson, J.B.; Arvanian, V.L. Neurotrophins and synaptic plasticity in the mammalian spinal cord. J. Physiol. 2001, 533, 91–97. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Wang, R.Z.; Wang, R.Z.; Li, G.L.; Wei, J.J.; Li, Z.J.; Feng, M.; Kang, J.; Du, W.C.; Ma, W.B.; et al. Transplantation of neural stem cells modified by human neurotrophin-3 promotes functional recovery after transient focal cerebral ischemia in rats. Neurosci. Lett. 2008, 444, 227–230. [Google Scholar] [CrossRef]
- Paterniti, I.; Impellizzeri, D.; Crupi, R.; Morabito, R.; Campolo, M.; Esposito, E.; Cuzzocrea, S. Molecular evidence for the involvement of PPAR-delta and PPAR-gamma in anti-inflammatory and neuroprotective activities of palmitoylethanolamide after spinal cord trauma. J. Neuroinflamm. 2013, 10, 20. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, N.Z.M.; Chiarotto, G.B.; Bernardes, D.; Kempe, P.R.G.; Oliveira, A.L.R. Neuroprotection by dimethyl fumarate following ventral root crush in C57BL/6J mice. Brain Res. Bull. 2020, 164, 184–197. [Google Scholar] [CrossRef] [PubMed]







| Antibody | Supplier | Host | Product Cat No. | Concentration |
|---|---|---|---|---|
| Synaptophysin | Synaptic Systems (Göttingen, Germany) | rabbit | NBP2-25170 | 1:1000 |
| Neurofilament | Millipore (Burlington, MA, USA) | rabbit | AB1989 | 1:2000 |
| NeuN | Millipore | mouse | MAB377 | 1:1500 |
| Alexa 488 | Jackson (West Grove, PA, USA) | rabbit | 711-545-152 | 1:400 |
| Alexa 594 | Jackson | mouse | 715-585-150 | 1:400 |
| Groups | Microenvironment | Motoneuron Coverage |
|---|---|---|
| VHL | 0.432 ± 0.031 | 0.412 ± 0.043 |
| DMF | 0.635 ± 0.037 | 0.541 ± 0.061 |
| FB+VHL | 0.750 ± 0.044 | 0.738 ± 0.043 |
| FB+DMF | 0.801 ± 0.040 | 0.741 ± 0.027 |
| Groups | Neuronal Perimeter (μm) | Total Synapse Coverage (%) | Number of Buttons/100 μm of Neuronal Membrane |
|---|---|---|---|
| CTL | 140.0 ± 7.2 | 63.03 ± 1.82 | 40.36 ± 2.05 |
| VHL | 105.0 ± 9.7 | 43.06 ± 4.38 | 23.22 ± 2.37 |
| DMF | 135.1 ± 5.2 | 51.7 ± 3.72 | 30.80 ± 2.75 |
| FB+VHL | 148.9 ± 3.1 | 64.87 ± 1.51 | 36.20 ± 1.26 |
| FB+DMF | 141.5 ± 4.2 | 63.32 ± 2.24 | 35.64 ± 1.32 |
| Groups | Total Synaptic Coverage (%) | Number of Boutons/100 μm | ||||
|---|---|---|---|---|---|---|
| F | S | C | F | S | C | |
| CTL | 51.98 ± 1.95 | 7.10 ± 0.89 | 3.94 ± 0.66 | 35.55 ± 2.21 | 3.72 ± 0.46 | 1.45 ± 0.15 |
| VHL | 36.08 ± 4.20 | 1.63 ± 0.86 | 5.34 ± 1.21 | 23.80 ± 3.14 | 0.80 ± 0.24 | 1.50 ± 0.26 |
| DMF | 40.13 ± 2.82 | 6.33 ± 1.61 | 5.22 ± 1.30 | 26.30 ± 2.08 | 2.55 ± 0.66 | 1.40 ± 0.22 |
| FB+VHL | 54.14 ± 1.66 | 4.54 ± 0.79 | 6.17 ± 0.73 | 32.40 ± 1.27 | 2.20 ± 0.43 | 1.60 ± 0.16 |
| FB+DMF | 43.35 ± 3.42 | 5.12 ± 0.87 | 5.14 ± 0.68 | 26.45 ± 2.06 | 2.60 ± 0.38 | 1.35 ± 0.13 |
| Groups | Total Synaptic Coverage (%) | Number of Buttons/100 μm of Neuronal Membrane | Number of Intervals |
|---|---|---|---|
| CTL | 63.03 ± 1.82 | 40.36 ± 2.05 | 44.73 ± 2.05 |
| VHL | 43.06 ± 4.38 | 23.22 ± 2.37 | 24.30 ± 3.40 |
| FB+DMF | 30.96 ± 3.31 | 17.83 ± 2.38 | 20.17 ± 1.90 |
| Groups | Neurofilament Immunostaining | Myelinated Axon Counting | Total Nerve Area (μm2) |
|---|---|---|---|
| CTL | 23,105 ± 533 | 1064 ± 84 | 257,097 ± 15,843 |
| VHL | 15,526 ± 642 | 756 ± 36 | 286,198 ± 29,407 |
| DMF | 17,021 ± 1254 | 876 ± 62 | 264,682 ± 10,953 |
| FB+VHL | 19,361 ± 362 | 893 ± 35 | 284,019 ± 13,227 |
| FB+DMF | 22,052 ± 642 | 1041 ± 46 | 239,858 ± 18,166 |
| Groups | FD (μm) | AD (μm) | MST (μm) | ‘g’ Ratio |
|---|---|---|---|---|
| CTL | 6.193 ± 0.033 | 4.658 ± 0.026 | 0.767 ± 0.004 | 0.750 ± 0.0005 |
| VHL | 6.418 ± 0.043 | 4.974 ± 0.036 | 0.721 ± 0.004 | 0.768 ± 0.0008 |
| DMF 15 | 6.237 ± 0.035 | 4.670 ± 0.028 | 0.783 ± 0.004 | 0.746 ± 0.0007 |
| FB+VHL | 6.199 ± 0.038 | 4.673 ± 0.030 | 0.762 ± 0.004 | 0.749 ± 0.0007 |
| FB+DMF | 5.997 ± 0.033 | 4.462 ± 0.026 | 0.767 ± 0.004 | 0.739 ± 0.0006 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kempe, P.R.G.; de Castro, M.V.; Khuriyeh, V.C.; Barraviera, B.; Ferreira, R.S., Jr.; de Oliveira, A.L.R. Ultrastructural Evidence of Synapse Preservation and Axonal Regeneration Following Spinal Root Repair with Fibrin Biopolymer and Therapy with Dimethyl Fumarate. Polymers 2023, 15, 3171. https://doi.org/10.3390/polym15153171
Kempe PRG, de Castro MV, Khuriyeh VC, Barraviera B, Ferreira RS Jr., de Oliveira ALR. Ultrastructural Evidence of Synapse Preservation and Axonal Regeneration Following Spinal Root Repair with Fibrin Biopolymer and Therapy with Dimethyl Fumarate. Polymers. 2023; 15(15):3171. https://doi.org/10.3390/polym15153171
Chicago/Turabian StyleKempe, Paula Regina Gelinski, Mateus Vidigal de Castro, Victor Campos Khuriyeh, Benedito Barraviera, Rui Seabra Ferreira, Jr., and Alexandre Leite Rodrigues de Oliveira. 2023. "Ultrastructural Evidence of Synapse Preservation and Axonal Regeneration Following Spinal Root Repair with Fibrin Biopolymer and Therapy with Dimethyl Fumarate" Polymers 15, no. 15: 3171. https://doi.org/10.3390/polym15153171