Influence of Coffee Variety and Processing on the Properties of Parchments as Functional Bioadditives for Biobased Poly(butylene succinate) Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Matrix
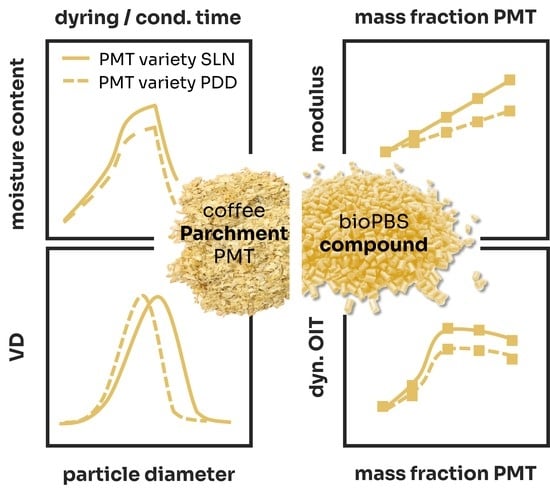
2.2. Coffee Parchments
2.3. Coffee Parchment Pretreatment and Analysis
2.4. Composite Processing
2.5. Composite Analysis
3. Results and Discussion
3.1. Coffee Parchments
3.2. Composites Based on BioPBS and PMT
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lachenmeier, D.W.; Teipel, J.; Scharinger, A.; Kuballa, T.; Walch, S.G.; Grosch, F.; Bunzel, M.; Okaru, A.O.; Schwarz, S. Fully Automated Identification of Coffee Species and Simultaneous Quantification of Furfuryl Alcohol Using NMR Spectroscopy. J. AOAC Int. 2020, 103, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Klingel, T.; Kremer, J.I.; Gottstein, V.; de Rezende, T.R.; Schwarz, S.; Lachenmeier, D.W. A Review of Coffee By-Products Including Leaf, Flower, Cherry, Husk, Silver Skin, and Spent Grounds as Novel Foods within the European Union. Foods 2020, 9, 665. [Google Scholar] [CrossRef] [PubMed]
- Iriondo-DeHond, A.; Iriondo-DeHond, M.; del Castillo, M.D. Applications of Compounds from Coffee Processing by-Products. Biomolecules 2020, 10, 1219. [Google Scholar] [CrossRef] [PubMed]
- Janissen, B.; Huynh, T. Chemical composition and value-adding applications of coffee industry by-products: A review. Resour. Conserv. Recycl. 2018, 128, 110–117. [Google Scholar] [CrossRef]
- Hejna, A. Potential applications of by-products from the coffee industry in polymer technology—Current state and perspectives. Waste Manag. 2021, 121, 296–330. [Google Scholar] [CrossRef]
- Oliveira, G.; Passos, C.P.; Ferreira, P.; Coimbra, M.A.; Gonçalves, I. Coffee By-Products and Their Suitability for Developing Active Food Packaging Materials. Foods 2021, 10, 683. [Google Scholar] [CrossRef]
- Rotta, N.M.; Curry, S.; Han, J.; Reconco, R.; Spang, E.; Ristenpart, W.; Donis-González, I.R. A comprehensive analysis of operations and mass flows in postharvest processing of washed coffee. Resour. Conserv. Recycl. 2021, 170, 105554. [Google Scholar] [CrossRef]
- Fernandes, A.; Mello, F.; Filho, S.T.; Carpes, R.; Honório, J.; Marques, M.; Felzenszwalb, I.; Ferraz, E. Impacts of discarded coffee waste on human and environmental health. Ecotoxicol. Environ. Saf. 2017, 141, 30–36. [Google Scholar] [CrossRef]
- Zarrelli, A.; DellaGreca, M.; Iesce, M.R.; Lavorgna, M.; Temussi, F.; Schiavone, L.; Criscuolo, E.; Parrella, A.; Previtera, L.; Isidori, M. Ecotoxicological evaluation of caffeine and its derivatives from a simulated chlorination step. Sci. Total Environ. 2014, 470–471, 453–458. [Google Scholar] [CrossRef] [Green Version]
- Mendoza Martinez, C.L.; Alves Rocha, E.P.; Oliveira Carneiro, A.D.C.; Borges Gomes, F.J.; Ribas Batalha, L.A.; Vakkilainen, E.; Cardoso, M. Characterization of residual biomasses from the coffee production chain and assessment the potential for energy purposes. Biomass Bioenergy 2019, 120, 68–76. [Google Scholar] [CrossRef]
- Bekalo, S.A.; Reinhardt, H.-W. Fibers of coffee husk and hulls for the production of particleboard. Mater. Struct. 2010, 43, 1049–1060. [Google Scholar] [CrossRef]
- Reis, R.S.; Tienne, L.G.; Souza, D.D.H.; Marques, M.D.F.V.; Monteiro, S.N. Characterization of coffee parchment and innovative steam explosion treatment to obtain microfibrillated cellulose as potential composite reinforcement. J. Mater. Res. Technol. 2020, 9, 9412–9421. [Google Scholar] [CrossRef]
- Estrada-Bahena, E.; Salazar, R.; Ramírez, M.; Moreno-Godínez, M.; Jiménez-Hernández, J.; Romero-Ramírez, Y.; González-Cortázar, M.; Alvarez-Fitz, P. Influence of water activity on physical properties, fungal growth, and ochratoxin A production in dry cherries and green-coffee beans. J. Food Process. Preserv. 2022, 46, e16226. [Google Scholar] [CrossRef]
- Pardo, E.; Marín, S.; Ramos, A.J.; Sanchis, V. Effect of water activity and temperature on mycelial growth and ochratoxin a production by isolates of Aspergillus ochraceus on irradiated green coffee beans. J. Food Prot. 2005, 68, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Mirón-Mérida, V.A.; Yáñez-Fernández, J.; Montañez-Barragán, B.; Barragán Huerta, B.E. Valorization of coffee parchment waste (Coffea arabica) as a source of caffeine and phenolic compounds in antifungal gellan gum films. LWT 2019, 101, 167–174. [Google Scholar] [CrossRef]
- Littardi, P.; Rinaldi, M.; Grimaldi, M.; Cavazza, A.; Chiavaro, E. Effect of Addition of Green Coffee Parchment on Structural, Qualitative and Chemical Properties of Gluten-Free Bread. Foods 2021, 10, 5. [Google Scholar] [CrossRef]
- Skřivan, P.; Sluková, M.; Jurkaninová, L.; Švec, I. Preliminary Investigations on the Use of a New Milling Technology for Obtaining Wholemeal Flours. Appl. Sci. 2021, 11, 6138. [Google Scholar] [CrossRef]
- Niemi, P.; Faulds, C.B.; Sibakov, J.; Holopainen, U.; Poutanen, K.; Buchert, J. Effect of a milling pre-treatment on the enzymatic hydrolysis of carbohydrates in brewer’s spent grain. Bioresour. Technol. 2012, 116, 155–160. [Google Scholar] [CrossRef]
- Goula, A.; Thymiatis, K.; Kaderides, K. Valorization of grape pomace: Drying behavior and ultrasound extraction of phenolics. Food Bioprod. Process. 2016, 100, 132–144. [Google Scholar] [CrossRef]
- Guaita, M.; Panero, L.; Motta, S.; Mangione, B.; Bosso, A. Effects of high-temperature drying on the polyphenolic composition of skins and seeds from red grape pomace. LWT 2021, 145, 111323. [Google Scholar] [CrossRef]
- Aliotta, L.; Seggiani, M.; Lazzeri, A.; Gigante, V.; Cinelli, P. A Brief Review of Poly (Butylene Succinate) (PBS) and Its Main Copolymers: Synthesis, Blends, Composites, Biodegradability, and Applications. Polymers 2022, 14, 844. [Google Scholar] [CrossRef]
- Kanemura, C.; Nakashima, S.; Hotta, A. Mechanical properties and chemical structures of biodegradable poly(butylene-succinate) for material reprocessing. Polym. Degrad. Stab. 2012, 97, 972–980. [Google Scholar] [CrossRef]
- Rafiqah, S.A.; Khalina, A.; Harmaen, A.S.; Tawakkal, I.A.; Zaman, K.; Asim, M.; Nurrazi, M.; Lee, C.H. A Review on Properties and Application of Bio-Based Poly(Butylene Succinate). Polymers 2021, 13, 1436. [Google Scholar] [CrossRef]
- Rizzarelli, P.; Carroccio, S. Thermo-oxidative processes in biodegradable poly(butylene succinate). Polym. Degrad. Stab. 2009, 94, 1825–1838. [Google Scholar] [CrossRef]
- Brito, J.; Hlushko, H.; Abbott, A.; Aliakseyeu, A.; Hlushko, R.; Sukhishvili, S.A. Integrating Antioxidant Functionality into Polymer Materials: Fundamentals, Strategies, and Applications. ACS Appl. Mater. Interfaces 2021, 13, 41372–41395. [Google Scholar] [CrossRef] [PubMed]
- Hallstein, J.; Gomoll, A.; Lieske, A.; Büsse, T.; Balko, J.; Brüll, R.; Malz, F.; Metzsch-Zilligen, E.; Pfaendner, R.; Zehm, D. Unraveling the cause for the unusual processing behavior of commercial partially bio-based poly(butylene succinates) and their stabilization. J. Appl. Polym. Sci. 2021, 138, 50669–50684. [Google Scholar] [CrossRef]
- Zimmermann, L.; Dombrowski, A.; Völker, C.; Wagner, M. Are bioplastics and plant-based materials safer than conventional plastics? In vitro toxicity and chemical composition. Environ. Int. 2020, 145, 106066. [Google Scholar] [CrossRef] [PubMed]
- Boncler, M.; Golanski, J.; Lukasiak, M.; Redzynia, M.; Dastych, J.; Watala, C. A new approach for the assessment of the toxicity of polyphenol-rich compounds with the use of high content screening analysis. PLoS ONE 2017, 12, e0180022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- LIczbiński, P.; Bukowska, B. Tea and coffee polyphenols and their biological properties based on the latest in vitro investigations. Ind. Crops Prod. 2022, 175, 114265. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Hiller, B.T.; Azzi, J.L.; Rennert, M. Improvement of the Thermo-Oxidative Stability of Biobased Poly(butylene succinate) (PBS) Using Biogenic Wine by-Products as Sustainable Functional Fillers. Polymers 2023, 15, 2533. [Google Scholar] [CrossRef]
- Miyata, T.; Masuko, T. Crystallization behaviour of poly(tetramethylene succinate). Polymer 1998, 39, 1399–1404. [Google Scholar] [CrossRef]
- Iriondo-DeHond, A.; Aparicio García, N.; Fernandez-Gomez, B.; Guisantes-Batan, E.; Velázquez Escobar, F.; Blanch, G.P.; Velázquez Escobar, M.I.; Sanchez-Fortun, S.; del Castillo, M.D. Validation of coffee by-products as novel food ingredients. Innov. Food Sci. Emerg. Technol. 2019, 51, 194–204. [Google Scholar] [CrossRef] [Green Version]
- Pereira, P.V.; Silveira, D.L.; Schwan, R.F.; Silva, S.A.; Coelho, J.M.; Bernardes, P.C. Effect of altitude and terrain aspect on the chemical composition of Coffea canephora cherries and sensory characteristics of the beverage. J. Sci. Food Agric. 2021, 101, 2570–2575. [Google Scholar] [CrossRef]
- Benitez, V.; Rebollo-Hernanz, M.; Hernanz, S.; Chantres, S.; Aguilera, Y.; Martin-Cabrejas, M.A. Coffee parchment as a new dietary fiber ingredient: Functional and physiological characterization. Food Res. Int. 2019, 122, 105–113. [Google Scholar] [CrossRef]
- Atanda, S.; Pessu, P.O.; Agoda, S.; Isong, I.U.; Adekalu, O.A.; Echendu, M.A.; Falade, T.C. Fungi and mycotoxins in stored foods. Afr. J. Microbiol. Res. 2011, 5, 4374–4383. [Google Scholar] [CrossRef]
- Vijayalaxmi, S.; Jayalakshmi, S.K.; Sreeramulu, K. Polyphenols from different agricultural residues: Extraction, identification and their antioxidant properties. J. Food Sci. Technol. 2015, 52, 2761–2769. [Google Scholar] [CrossRef] [Green Version]
- Aguilera, Y.; Rebollo-Hernanz, M.; Cañas, S.; Taladrid, D.; Martín-Cabrejas, M.A. Response surface methodology to optimise the heat-assisted aqueous extraction of phenolic compounds from coffee parchment and their comprehensive analysis. Food Funct. 2019, 10, 4739–4750. [Google Scholar] [CrossRef]
- Cangussu, L.B.; Melo, J.C.; Franca, A.S.; Oliveira, L.S. Chemical Characterization of Coffee Husks, a by-Product of Coffea arabica Production. Foods 2021, 10, 3125. [Google Scholar] [CrossRef]
- Salem, F.H.; Achir, N.; Sieczkowski, N.; Boulanger, R.; Collignan, A. Modelling the transfer and degradation kinetics of aroma compounds from liquid media into coffee beans during simulated wet processing conditions. J. Food Eng. 2023, 343, 111303. [Google Scholar] [CrossRef]
- Benítez, V.; Rebollo-Hernanz, M.; Aguilera, Y.; Bejerano, S.; Cañas, S.; Martín-Cabrejas, M.A. Extruded coffee parchment shows enhanced antioxidant, hypoglycaemic, and hypolipidemic properties by releasing phenolic compounds from the fibre matrix. Food Funct. 2021, 12, 1097–1110. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Wu, H.; Viejo, C.G.; Dunshea, F.R.; Suleria, H.A.R. Effects of postharvest processing on aroma formation in roasted coffee—A review. Int. J. Food Sci. Technol. 2023, 58, 1007–1027. [Google Scholar] [CrossRef]
- Gancarz, M.; Dobrzański, B.; Malaga-Toboła, U.; Tabor, S.; Combrzyński, M.; Ćwikła, D.; Strobel, W.; Oniszczuk, A.; Karami, H.; Darvishi, Y.; et al. Impact of Coffee Bean Roasting on the Content of Pyridines Determined by Analysis of Volatile Organic Compounds. Molecules 2022, 27, 1559. [Google Scholar] [CrossRef]
- Javadi, A.; Srithep, Y.; Pilla, S.; Lee, J.; Gong, S.; Turng, L.-S. Processing and characterization of solid and microcellular PHBV/coir fiber composites. Mater. Sci. Eng. C 2010, 30, 749–757. [Google Scholar] [CrossRef]
- Reis, K.C.; Pereira, L.; Melo, I.C.N.A.; Marconcini, J.M.; Trugilho, P.F.; Tonoli, G.H.D. Particles of Coffee Wastes as Reinforcement in Polyhydroxybutyrate (PHB) Based Composites. Mater. Res. 2015, 18, 546–552. [Google Scholar] [CrossRef] [Green Version]
- Gowman, A.; Wang, T.; Rodriguez-Uribe, A.; Mohanty, A.K.; Misra, M. Bio-poly(butylene succinate) and Its Composites with Grape Pomace: Mechanical Performance and Thermal Properties. ACS Omega 2018, 3, 15205–15216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Opitz, S.E.W.; Smrke, S.; Goodman, B.A.; Keller, M.; Schenker, S.; Yeretzian, C. Antioxidant Generation during Coffee Roasting: A Comparison and Interpretation from Three Complementary Assays. Foods 2014, 3, 586–604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masek, A. Flavonoids as Natural Stabilizers and Color Indicators of Ageing for Polymeric Materials. Polymers 2015, 7, 1125–1144. [Google Scholar] [CrossRef] [Green Version]
- Georgousopoulou, I.-N.; Vouyiouka, S.; Dole, P.; Papaspyrides, C.D. Thermo-mechanical degradation and stabilization of poly(butylene succinate). Polym. Degrad. Stab. 2016, 128, 182–192. [Google Scholar] [CrossRef]
- Dintcheva, N.; D’Anna, F. Anti-/Pro-Oxidant Behavior of Naturally Occurring Molecules in Polymers and Biopolymers: A Brief Review. ACS Sustain. Chem. Eng. 2019, 7, 12656–12670. [Google Scholar] [CrossRef]















| H2O (%) | aw (-) | D97 | TPC | AADPPH | AAABTS | |||
|---|---|---|---|---|---|---|---|---|
| native * | DG ** | native | DG | µm | mg GAE/g dw | TEAC (mmol TE/100 g dw) | ||
| PMT-PDD | 10.3 | 1.79 | 0.47 | 0.14 | 93.5 | 8.46 | 2.75 | 5.36 |
| PMT-SLN | 7.0 | 1.16 | 0.32 | 0.12 | 168.2 | 7.47 | 0.77 | 2.94 |
| Ta | T10% | DTG | ΔmW | ΔmHC | ΔmHC | ΔmC | ΔmL | mresidual | |
|---|---|---|---|---|---|---|---|---|---|
| °C | °C | °C | % | % | % | % | % | % | |
| PMT-SLN | 302.5 | 285.5 | 364.4 | 1.0 | 0.5 | 15.4 | 54.4 | 6.79 | 20.9 |
| PMT-PDD | 272.7 | 242.6 | 329.6 | 1.6 | 6.7 | 10.4 | 40.2 | 13.9 | 26.3 |
| PMT-SLN Composites | PMT-PDD Composites | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mass Fraction | Tg | Tm | Xc | T10% | Ton | DTG | Tg | Tm | Xc | T10% | Ton | DTG |
| wt.-% | °C | °C | % | °C | °C | °C | °C | °C | % | °C | °C | °C |
| 0 | −25.0 | 116.5 | 34 | 361.7 | 367.5 | 398.8 | −25.0 | 116.5 | 34 | 361.7 | 367.5 | 398.8 |
| 5 | −27.4 | 117.0 | 33 | - | - | - | −23.8 | 118.4 | 35 | - | - | - |
| 10 | −26.9 | 116.5 | 35 | 356.5 | 367.5 | 396.1 | −31.3 | 117.7 | 36 | 351.7 | 362.8 | 390.9 |
| 15 | −27.6 | 117.3 | 34 | - | - | - | −27.5 | 117.1 | 38 | - | - | - |
| 20 | −28.5 | 116.1 | 34 | 343.7 | 360.9 | 394.4 | −27.8 | 116.6 | 36 | 341.5 | 358.7 | 391.5 |
| PMT-SLN | PMT-PDD | |||||
|---|---|---|---|---|---|---|
| Filler Content | Ox. T10% | Ox. Ton | Ox. DTG | Ox. T10% | Ox. Ton | Ox. DTG |
| wt.-% | °C | °C | °C | °C | °C | °C |
| 0 | 320.4 | 347.9 | 397.1 | 320.4 | 347.9 | 397.1 |
| 10 | 353.2 | 365.2 | 392.6 | 347.0 | 358.3 | 385.9 |
| 20 | 339.1 | 360.6 | 392.8 | 340.1 | 355.4 | 386.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rennert, M.; Hiller, B.T. Influence of Coffee Variety and Processing on the Properties of Parchments as Functional Bioadditives for Biobased Poly(butylene succinate) Composites. Polymers 2023, 15, 2985. https://doi.org/10.3390/polym15142985
Rennert M, Hiller BT. Influence of Coffee Variety and Processing on the Properties of Parchments as Functional Bioadditives for Biobased Poly(butylene succinate) Composites. Polymers. 2023; 15(14):2985. https://doi.org/10.3390/polym15142985
Chicago/Turabian StyleRennert, Mirko, and Benedikt T. Hiller. 2023. "Influence of Coffee Variety and Processing on the Properties of Parchments as Functional Bioadditives for Biobased Poly(butylene succinate) Composites" Polymers 15, no. 14: 2985. https://doi.org/10.3390/polym15142985