Incorporation of Fly Ash in Flame-Retardant Systems of Biopolyesters
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
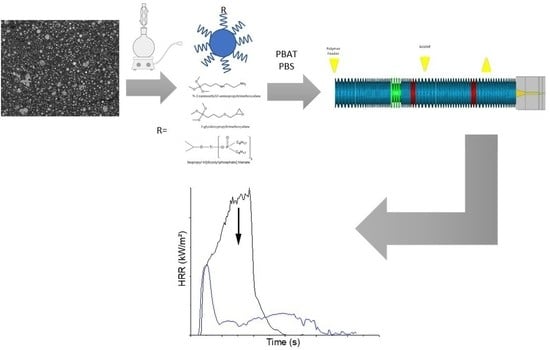
2.2.1. Processing of Compositions
2.2.2. Surface Modifications
2.2.3. Thermal Stability and Fire Reaction
2.2.4. Study of Microstructures and Composition of Residues
3. Results
3.1. Thermal Analysis
3.2. Pyrolysis Combustion Flow Calorimeter
3.3. Cone Calorimeter Tests
3.4. SEM Observations and an X-Microanalysis of Cone Calorimeter Residues
3.5. X-Ray Diffractometry of the Residues
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Kabir, I.I.; Sorrell, C.C.; Mada, M.R.; Cholake, S.T.; Bandyopadhyay, S. General model for comparative tensile mechanical properties of composites fabricated from fly ash and virgin/recycled high-density polyethylene. Polym. Eng. Sci. 2016, 56, 1096–1108. [Google Scholar] [CrossRef]
- Hanif, A.; Diao, S.; Lu, Z.; Fan, T.; Li, Z. Green lightweight cementitious composite incorporating aerogels and fly ash cenospheres—Mechanical and thermal insulating properties. Constr. Build. Mater. 2016, 116, 422–430. [Google Scholar] [CrossRef]
- Kolay, P.K.; Bhusal, S. Recovery of hollow spherical particles with two different densities from coal fly ash and their characterization. Fuel 2014, 117, 118–124. [Google Scholar] [CrossRef]
- Ranjbar, N.; Kuenzel, C. Cenospheres: A review. Fuel 2017, 207, 1–12. [Google Scholar] [CrossRef]
- Yao, Z.T.; Ji, X.S.; Sarker, P.K.; Tang, J.H.; Ge, L.Q.; Xia, M.S.; Xi, Y.Q. A comprehensive review on the applications of coal fly ash. Earth-Sci. Rev. 2015, 141, 105–121. [Google Scholar] [CrossRef] [Green Version]
- Varughese, K.T.; Chaturvedi, B.K. Fly ash as fine aggregate in polyester based polymer concrete. Cem. Concr. Compos. 1996, 18, 105–108. [Google Scholar] [CrossRef]
- Fomenko, E.V.; Anshits, N.N.; Anshits, A.G. The Composition, Structure, and Helium Permeability of Glass-Crystalline Shells of Cenospheres. Glass Phys. Chem. 2019, 45, 36–46. [Google Scholar] [CrossRef]
- Zierold, K.M.; Odoh, C. A review on fly ash from coal-fired power plants: Chemical composition, regulations, and health evidence. Rev. Environ. Health 2020, 35, 401–418. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, S.; Mehrotra, S.P. Towards sustainable solutions for fly ash through mechanical activation. Resour. Conserv. Recycl. 2007, 52, 157–179. [Google Scholar] [CrossRef]
- Nath, D.C.D.; Bandyopadhyay, S.; Rider, J.; Yu, A.; Blackburn, D.; White, C. Study of dynamic mechanical properties and morphological behaviours of fly ash reinforced polypropylene composites. Macromol. Res. 2011, 19, 338–344. [Google Scholar] [CrossRef]
- Satapathy, S.; Kothapalli, R.V.S. Mechanical, Dynamic Mechanical and Thermal Properties of Banana Fiber/Recycled High Density Polyethylene Biocomposites Filled with Flyash Cenospheres. J. Polym. Environ. 2018, 26, 200–213. [Google Scholar] [CrossRef]
- Ogah, A.O.; Afiukwa, J.N. Characterization and comparison of mechanical behavior of agro fiber-filled high-density polyethylene bio-composites. J. Reinf. Plast. Compos. 2014, 33, 37–46. [Google Scholar] [CrossRef]
- Das, A.; Satapathy, B.K. Structural, thermal, mechanical and dynamic mechanical properties of cenosphere filled polypropylene composites. Mater. Des. 2011, 32, 1477–1484. [Google Scholar] [CrossRef]
- Deepthi, M.V.; Sharma, M.; Sailaja, R.R.N.; Anantha, P.; Sampathkumaran, P.; Seetharamu, S. Mechanical and thermal characteristics of high density polyethylene-fly ash Cenospheres composites. Mater. Des. 2010, 31, 2051–2060. [Google Scholar] [CrossRef]
- Satapathy, S.; Kothapalli, R.V.S. Influence of fly ash cenospheres on performance of coir fiber-reinforced recycled high-density polyethylene biocomposites. J. Appl. Polym. Sci. 2015, 132, 1–14. [Google Scholar] [CrossRef]
- Abu-Zahra, N.; Khoshnoud, P. Effect of Cenosphere Fly Ash on the Thermal, Mechanical, and Morphological Properties of Rigid PVC Foam Composites. J. Res. Updat. Polym. Sci. 2015, 4, 1–14. [Google Scholar] [CrossRef]
- Van der Merwe, E.M.; Mathebula, C.L.; Prinsloo, L.C. Characterization of the surface and physical properties of South African coal fly ash modified by sodium lauryl sulphate (SLS) for applications in PVC composites. Powder Technol. 2014, 266, 70–78. [Google Scholar] [CrossRef] [Green Version]
- Bonda, S.; Mohanty, S.; Nayak, S.K. Viscoelastic, mechanical, and thermal characterization of fly ash-filled ABS composites and comparison of fly ash surface treatments. Polym. Compos. 2012, 33, 22–34. [Google Scholar] [CrossRef]
- Xuan, Y.; Huajin, T.; Zhiwen, Q. Effect of mixing time of coupling agent on properties of 3D printing prototype of fly ash filled ABS. IOP Conf. Ser. Mater. Sci. Eng. 2019, 631, 032025. [Google Scholar] [CrossRef]
- Chaowasakoo, T.; Sombatsompop, N. Mechanical and morphological properties of fly ash/epoxy composites using conventional thermal and microwave curing methods. Compos. Sci. Technol. 2007, 67, 2282–2291. [Google Scholar] [CrossRef]
- Sim, J.; Kang, Y.; Kim, B.J.; Park, Y.H.; Lee, Y.C. Preparation of Fly Ash/Epoxy Composites and Its Effects on Mechanical Properties. Polymers 2020, 12, 79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guhanathan, S.; Saroja Devi, M. Studies on interface in polyester/fly-ash particulate composites. Compos. Interfaces 2004, 11, 43–66. [Google Scholar] [CrossRef]
- Guhanathan, S.; Devi, M.S.; Murugesan, V. Effect of coupling agents on the mechanical properties of fly ash/polyester particulate composites. J. Appl. Polym. Sci. 2001, 82, 1755–1760. [Google Scholar] [CrossRef]
- Psyanchin, A.A.; Zakharova, E.M.; Khusnullin, A.G.; Zakharov, V.P. Development of a spatially structured polymeric matrix under UV irradiation of polylactide-based composites filled with aluminosilicate microspheres. INCAS Bull. 2021, 13, 159–167. [Google Scholar] [CrossRef]
- Chand, N.; Sharma, P.; Fahim, M. Correlation of mechanical and tribological properties of organosilane modified cenosphere filled high density polyethylene. Mater. Sci. Eng. A 2010, 527, 5873–5878. [Google Scholar] [CrossRef]
- Padhi, S.; Priyadarsini Jena, D.; Nayak, N.C. Dielectric behaviour of ethylene vinyl acetate/cenosphere composites. Mater. Today Proc. 2020, 30, 355–359. [Google Scholar] [CrossRef]
- Alkadasi, N.A.N.; Kapadi, U.R.; Hundiwale, D.G. Effect of titanate coupling agent on the mechanical properties of clay-filled polybutadiene rubber. J. Appl. Polym. Sci. 2004, 93, 1299–1304. [Google Scholar] [CrossRef]
- Puri, R.G.; Khanna, A.S. Effect of cenospheres on the char formation and fire protective performance of water-based intumescent coatings on structural steel. Prog. Org. Coat. 2016, 92, 8–15. [Google Scholar] [CrossRef]
- Bumbudsanpharoke, N.; Wongphan, P.; Promhuad, K.; Leelaphiwat, P.; Harnkarnsujarit, N. Morphology and permeability of bio-based poly(butylene adipate-co-terephthalate) (PBAT), poly(butylene succinate) (PBS) and linear low-density polyethylene (LLDPE) blend films control shelf-life of packaged bread. Food Control 2022, 132, 108541. [Google Scholar] [CrossRef]
- Sonnier, R.; Taguet, A.; Ferry, L.; Lopez-Cuesta, J.-M. Towards Bio-Based Flame Retardant Polymers; Springer Briefs in Molecular Science; Springer International Publishing: Cham, Switzerland, 2018; ISBN 978-3-319-67082-9. [Google Scholar]
- Yang, W.; Tawiah, B.; Yu, C.; Qian, Y.-F.; Wang, L.-L.; Yuen, A.C.-Y.; Zhu, S.-E.; Hu, E.-Z.; Chen, T.B.-Y.; Yu, B.; et al. Manufacturing, mechanical and flame retardant properties of poly(lactic acid) biocomposites based on calcium magnesium phytate and carbon nanotubes. Compos. Part A Appl. Sci. Manuf. 2018, 110, 227–236. [Google Scholar] [CrossRef]
- Xiao, F.; Fontaine, G.; Bourbigot, S. Recent developments in fire retardancy of polybutylene succinate. Polym. Degrad. Stab. 2021, 183, 109466. [Google Scholar] [CrossRef]
- Ferry, L.; Dorez, G.; Taguet, A.; Otazaghine, B.; Lopez-Cuesta, J.M. Chemical modification of lignin by phosphorus molecules to improve the fire behavior of polybutylene succinate. Polym. Degrad. Stab. 2015, 113, 135–143. [Google Scholar] [CrossRef]
- Dorez, G.; Taguet, A.; Ferry, L.; Lopez Cuesta, J.-M. Phosphorous compounds as flame retardants for polybutylene succinate/flax biocomposite: Additive versus reactive route. Polym. Degrad. Stab. 2014, 102, 152–159. [Google Scholar] [CrossRef]
- Hu, C.; Bourbigot, S.; Delaunay, T.; Collinet, M.; Marcille, S.; Fontaine, G. Synthesis of isosorbide based flame retardants: Application for polybutylene succinate. Polym. Degrad. Stab. 2019, 164, 9–17. [Google Scholar] [CrossRef]
- Chen, S.; Wu, F.; Hu, Y.; Lin, S.; Yu, C.; Zhu, F.; Wang, X. A fully bio-based intumescent flame retardant for poly(butylene succinate). Mater. Chem. Phys. 2020, 252, 123222. [Google Scholar] [CrossRef]
- Kuan, C.-F.; Kuan, H.-C.; Ma, C.-C.M.; Chen, C.-H. Flame retardancy and nondripping properties of ammonium polyphosphate/poly(butylene succinate) composites enhanced by water crosslinking. J. Appl. Polym. Sci. 2006, 102, 2935–2945. [Google Scholar] [CrossRef]
- Liu, Y.-J.; Mao, L.; Fan, S. Preparation and study of intumescent flame retardant poly(butylene succinate) using MgAlZnFe-CO3 layered double hydroxide as a synergistic agent. J. Appl. Polym. Sci. 2014, 131, 1–10. [Google Scholar] [CrossRef]
- Dumazert, L.; Rasselet, D.; Pang, B.; Gallard, B.; Kennouche, S.; Lopez-Cuesta, J.-M. Thermal stability and fire reaction of poly(butylene succinate) nanocomposites using natural clays and FR additives. Polym. Adv. Technol. 2018, 29, 69–83. [Google Scholar] [CrossRef]
- Lyon, R.E.; Walters, R.N. Pyrolysis combustion flow calorimetry. J. Anal. Appl. Pyrolysis 2004, 71, 27–46. [Google Scholar] [CrossRef]
- Batistella, M.; Roux, J.-C.; le Saout, G.; Callone, E.; Diré, S.; Xenopoulos, C.; Lopez-Cuesta, J.-M. Use of fly ash as synergistic and reactive component of flame retardant system in polylactide. Polym. Degrad. Stab. 2023, 211, 110314. [Google Scholar] [CrossRef]














| Polymer | Zone 1 | Zone 2 | Zone 3 | Zone 4 | Zone 5 | Zone 5 | Zone 7 | Zone 8 | Zone 9 |
|---|---|---|---|---|---|---|---|---|---|
| PBS | 25 | 110 | 115 | 120 | 125 | 130 | 130 | 130 | 130 |
| PBAT | 60 | 90 | 160 | 180 | 180 | 180 | 180 | 180 | 180 |
| Formulation | PBAT | AP | MP | SP | SP-G | SP-T |
|---|---|---|---|---|---|---|
| PBAT | 100 | |||||
| PBAT-1 | 75 | 25 | ||||
| PBAT-2 | 75 | 18.75 | 6.25 | |||
| PBAT-3 | 75 | 18.75 | 6.25 | |||
| PBAT-4 | 75 | 18.75 | 6.25 | |||
| PBAT-5 | 75 | 12.5 | 12.5 | |||
| PBAT-6 | 75 | 12.5 | 12.5 | |||
| PBAT-7 | 75 | 12.5 | 12.5 | |||
| PBAT-8 | 75 | 16.67 | 8.33 | |||
| PBAT-9 | 75 | 12.5 | 6.25 | 6.25 | ||
| PBAT-10 | 75 | 12.5 | 6.25 | 6.25 | ||
| PBAT-11 | 75 | 12.5 | 6.25 | 6.25 |
| Formulation | PBS | AP | MP | SP | SP-A | SP-T |
|---|---|---|---|---|---|---|
| PBS | 100 | |||||
| PBS-1 | 75 | 25 | ||||
| PBS-2 | 75 | 18.75 | 6.25 | |||
| PBS-3 | 75 | 18.75 | 6.25 | |||
| PBS-4 | 75 | 18.75 | 6.25 | |||
| PBS-5 | 75 | 12.5 | 12.5 | |||
| PBS-6 | 75 | 12.5 | 12.5 | |||
| PBS-7 | 75 | 12.5 | 12.5 | |||
| PBS-8 | 75 | 16.67 | 8.33 | |||
| PBS-9 | 75 | 12.5 | 6.25 | 6.25 | ||
| PBS-10 | 75 | 12.5 | 6.25 | 6.25 | ||
| PBS-11 | 75 | 12.5 | 6.25 | 6.25 |
| Formulation | T5% (°C) | T20% (°C) | T50% (°C) | Residue at 750 °C (%) |
|---|---|---|---|---|
| PBAT | 382 | 397 | 410 | 7 |
| PBAT-1 | 357 | 381 | 393 | 18 |
| PBAT-2 | 354 | 370 | 382 | 18.4 |
| PBAT-3 | 352 | 368 | 382 | 19.4 |
| PBAT-4 | 356 | 376 | 388 | 25.5 |
| PBAT-5 | 357 | 374 | 388 | 26.5 |
| PBAT-6 | 355 | 375 | 390 | 27 |
| PBAT-7 | 360 | 378 | 390 | 28 |
| PBAT-8 | 346 | 374 | 394 | 16.9 |
| PBAT-9 | 347 | 377 | 388 | 23.9 |
| PBAT-10 | 338 | 370 | 383 | 16.6 |
| PBAT-11 | 338 | 370 | 384 | 16.8 |
| Formulation | T5% (°C) | T20% (°C) | T50% (°C) | Residue at 750 °C (%) |
|---|---|---|---|---|
| PBS | 364 | 389 | 404 | - |
| PBS-1 | 342 | 348 | 349 | 22 |
| PBS-2 | 339 | 347 | 348 | 25 |
| PBS-3 | 339 | 346 | 348 | 23.6 |
| PBS-4 | 338 | 346 | 347 | 23.4 |
| PBS-5 | 337 | 347 | 350 | 21.9 |
| PBS-6 | 338 | 349 | 351 | 22.1 |
| PBS-7 | 336 | 345 | 349 | 20.3 |
| PBS-8 | 332 | 347 | 357 | 16.3 |
| PBS-9 | 336 | 348 | 355 | 24.2 |
| PBS-10 | 336 | 348 | 353 | 23.2 |
| PBS-11 | 335 | 347 | 352 | 23.6 |
| Formulation | Temperature pHRR (°C) | pHRR (W/g) | THR (KJ/g) | HRC (J/g/°C) | % Residue |
|---|---|---|---|---|---|
| PBAT | 427 | 516 | 23.0 | 537 | 1.3 |
| PBAT-1 | 407 | 421 | 19.5 | 436 | 11.7 |
| PBAT-2 | 411 | 435 | 18.1 | 452 | 16.0 |
| PBAT-3 | 410 | 405 | 18.2 | 419 | 18.4 |
| PBAT-4 | 412 | 400 | 18.1 | 411 | 19.0 |
| PBAT-5 | 412 | 421 | 18.3 | 439 | 19.6 |
| PBAT-6 | 415 | 428 | 18.3 | 444 | 21.3 |
| PBAT-7 | 414 | 438 | 18.3 | 463 | 21.6 |
| PBAT-8 | 415 | 492 | 17.0 | 515 | 14.2 |
| PBAT-9 | 408 | 496 | 18.7 | 522 | 17.1 |
| PBAT-10 | 411 | 541 | 18.6 | 559 | 16.7 |
| PBAT-11 | 409 | 534 | 18.6 | 558 | 16.5 |
| Formulation | Temperature pHRR (°C) | pHRR (W/g) | THR (KJ/g) | HRC (J/g/°C) | % Residue |
|---|---|---|---|---|---|
| PBS | 425 | 463 | 20.7 | 474 | 0 |
| PBS-1 | 385 | 907 | 15.8 | 933 | 9.2 |
| PBS-2 | 389 | 696 | 16.0 | 717 | 19.2 |
| PBS-3 | 386 | 716 | 17.1 | 742 | 18.0 |
| PBS-4 | 388 | 570 | 16.6 | 587 | 18.7 |
| PBS-5 | 394 | 450 | 16.2 | 463 | 20.9 |
| PBS-6 | 389 | 654 | 16.5 | 672 | 19.4 |
| PBS-7 | 391 | 621 | 15.9 | 641 | 20.7 |
| PBS-8 | 399 | 482 | 15.3 | 496 | 15.7 |
| PBS-9 | 390 | 874 | 16.4 | 908 | 14.9 |
| PBS-10 | 392 | 765 | 16.7 | 788 | 13.9 |
| PBS-11 | 390 | 816 | 16.7 | 851 | 15.9 |
| Formulation | TTI (s) | pHRR (kW/m2) | THR (MJ/m2) | MAHRE (kW/m2) | Residue (%) | UL94 |
|---|---|---|---|---|---|---|
| PBAT | 71 ± 3 | 751 ± 60 | 93 ± 5 | 345 ± 50 | 8 ± 1 | NC |
| PBAT-1 | 59 ± 2 | 485 ± 5 | 91 ± 1 | 292 ± 6 | 19 ± 0.1 | V-2 |
| PBAT-2 | 61 ± 2 | 480 ± 70 | 94 ± 0.6 | 282 ± 35 | 19 ± 0.2 | V-2 |
| PBAT-3 | 64 ± 2 | 420 ± 20 | 98 ± 1 | 235 ± 13 | 20 ± 0.4 | V-2 |
| PBAT-4 | 57 ± 9 | 461 ± 10 | 93 ± 2 | 283 ± 7 | 20 ± 0.4 | V-2 |
| PBAT-5 | 59 ± 3 | 379 ± 8 | 98 ± 0.3 | 244 ± 3 | 21 | V-2 |
| PBAT-6 | 56 ± 6 | 393 ± 19 | 91 ± 0.3 | 262 ± 10 | 21 ± 0.4 | V-2 |
| PBAT-7 | 56 ± 2 | 409 ± 11 | 93 ± 1 | 280 ± 9 | 20 ± 0.2 | V-2 |
| PBAT-8 | 66 ± 3 | 432 ± 4 | 94 ± 1 | 246 ± 12 | 17 ± 0.1 | V-2 |
| PBAT-9 | 55 ± 1 | 366 ± 38 | 95 ± 2 | 159 ± 4 | 21 ± 0.2 | V-2 |
| PBAT-10 | 61 ± 2 | 342 ± 20 | 93± | 147 ± 12 | 21 ± 2 | V-2 |
| PBAT-11 | 60 ± 6 | 285 ± 28 | 94 ± 2 | 120 ± 3 | 21 ± 2 | V-2 |
| Formulation | TTI (s) | pHRR (kW/m2) | THR (MJ/m2) | MAHRE (kW/m2) | Residue (%) | UL94-V |
|---|---|---|---|---|---|---|
| PBS | 71 ± 4 | 698 ± 22 | 103 ± 1 | 314 ± 6 | 8 ± 0.5 | NC |
| PBS-1 | 71 ± 2 | 524 ± 15 | 92 ± 1 | 241 ± 2 | 22 ± 0.5 | V-2 |
| PBS-2 | 63 ± 1 | 483 ± 15 | 97 ± 1 | 225 ± 9 | 21 ± 0.1 | V-2 |
| PBS-3 | 68 ± 0.7 | 411 ± 6 | 97 ± 0.5 | 228 ± 3 | 20 ± 0.1 | V-2 |
| PBS-4 | 68 ± 4 | 410 ± 11 | 97 ± 1 | 226 ± 1 | 20 ± 0.1 | V-2 |
| PBS-5 | 68 ± 5 | 450 ± 6 | 96 ± 2 | 281 ± 8 | 21 ± 0.1 | V-2 |
| PBS-6 | 67 ± 5 | 434 ± 11 | 96 ± 1 | 272 ± 3 | 21 ± 0.2 | V-2 |
| PBS-7 | 65 ± 3 | 425 ± 8 | 97 ± 0.5 | 282 ± 5 | 20 ± 0.1 | V-2 |
| PBS-8 | 60 ± 1 | 419 ± 6 | 92 ± 1 | 307 ± 31 | 20 ± 0.3 | V-0 |
| PBS-9 | 61 ± 6 | 383 ± 16 | 98 ± 1 | 225 ± 15 | 19 ± 0.2 | V-0 |
| PBS-10 | 63 ± 2 | 375 ± 6 | 97± | 214 ± 4 | 19 ± 0.1 | V-0 |
| PBS-11 | 68 ± 2 | 338 ± 4 | 98 ± 1 | 213 ± 3 | 19 ± 0.1 | V-0 |
| Formulation | Ca (wt.%) | Al (wt.%) | Si (wt.%) | Fe (wt.%) | C (wt.%) | P (wt.%) | N (wt.%) | Phosphorus Balance (%) |
|---|---|---|---|---|---|---|---|---|
| PBS-1 | - | - | - | - | 34 | 24 | 4 | 65 |
| PBS-3 | 1 | 4 | 6 | 1 | 12 | 25 | 3 | 85 |
| PBS-8 | - | - | - | - | 23 | 28 | 4 | |
| PBS-9 | 1 | 5 | 7 | 1 | 16 | 21 | - | 78 |
| PBS-10 | 1 | 4 | 6 | 0.5 | 17 | 21 | - | 81 |
| PBS-11 | 1 | 4 | 6 | 0.5 | 16 | 22 | - | 84 |
| Formulation | Ca (wt.%) | Al (wt.%) | Si (wt.%) | Fe (wt.%) | C (wt.%) | P (wt.%) | N (wt.%) | Phosphorus Balance (%) |
|---|---|---|---|---|---|---|---|---|
| PBAT-1 | - | 3 | - | - | 25 | 26 | 2 | 53 |
| PBAT-3 | 0.5 | 5 | 8 | 0.5 | 11 | 23 | - | 82 |
| PBAT-8 | - | 0.5 | 0.2 | - | 8 | 29 | 4 | |
| PBAT-9 | 0.5 | 5 | 5 | 1 | 12 | 25 | - | 100 |
| PBAT-10 | 1 | 5 | 6 | 0.5 | 8 | 26 | - | 100 |
| PBAT-11 | 1 | 5 | 5 | 1 | 10 | 26 | - | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Batistella, M.; Roux, J.-C.; Masarra, N.-A.; le Saout, G.; Xenopoulos, C.; Lopez-Cuesta, J.-M. Incorporation of Fly Ash in Flame-Retardant Systems of Biopolyesters. Polymers 2023, 15, 2771. https://doi.org/10.3390/polym15132771
Batistella M, Roux J-C, Masarra N-A, le Saout G, Xenopoulos C, Lopez-Cuesta J-M. Incorporation of Fly Ash in Flame-Retardant Systems of Biopolyesters. Polymers. 2023; 15(13):2771. https://doi.org/10.3390/polym15132771
Chicago/Turabian StyleBatistella, Marcos, Jean-Claude Roux, Nour-Alhoda Masarra, Gwenn le Saout, Constantinos Xenopoulos, and José-Marie Lopez-Cuesta. 2023. "Incorporation of Fly Ash in Flame-Retardant Systems of Biopolyesters" Polymers 15, no. 13: 2771. https://doi.org/10.3390/polym15132771