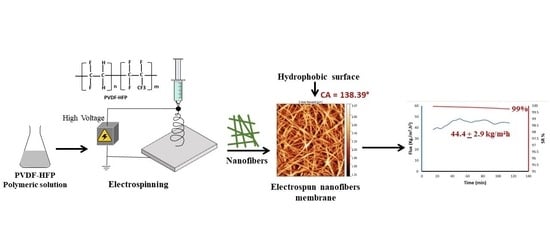
3.1. Dope Solution Characterizations
Hansen solubility parameters (HSP) are utilized to estimate the possibility of miscibility of two or more materials. Thus, the HSP can be written as:
where
δd,
δp, and
δh represent the dispersion force between molecules, polar cohesion, and hydrogen bond force parameters, respectively; these are listed in
Table 2 for the solvents and the polymer used in this study.
A is a symbol of the polymer (PVDF-HFP) and
B is a symbol of the solvent or nonsolvent.
RA-B represents the distance from the solvent location to the center of the solute molecule. Thus, it can be compared with the solubility radius of a solute (
R0), such as a polymer in this case, which creates a new term named the relative energy density (
RED).
The relative energy density (
RED) gives the radius of interaction that describes the relative compatibility between two components, and it can be written as [
26]:
There are three cases of RED results:
- i.
If RED < 1, it means the polymer will not dissolve.
- ii.
If RED > 1, it means the polymer will dissolve.
- iii.
If RED = 1, it means the polymer may swell.
Indeed, all binary solvents’
RED values were less than one, which confirms the ability of solvents to dissolve the polymer. The production of the membranes in electrospinning depends on several factors, including the rate of solvent evaporation, which is measured using the vapor pressure concept. Vapor pressure always indicates the volatility of the solvent and can be calculated according to Raoult’s law by the following equation:
The purpose of utilizing binary solvents is to control the vapor pressure, which in turn controls the solvent’s evaporation rate during the electrospinning process. Since acetone has a high vapor pressure (25 kPa), it is usually mixed with the major solvent to accelerate solvent evaporation and fabricate a dry membrane. The high vapor pressure of the binary solvents ensures the possibility of using dope solutions in electrospinning and producing a dry membrane. The vapor pressure of the binary solvents (i.e., DMF/acetone) is listed in
Table 3; it increased with the increasing acetone weight fraction.
The viscosity and surface tension of solutions are critical properties in the electrospinning process, so they should be in an appropriate range where the process cannot proceed under or above this range. At high viscosity, polymer extrusion is impossible, while at low viscosity, the droplets of the polymer may interrupt. The surface tension usually determines the voltage used in electrospinning that is required to be overcome. It is determined by solvent ratios and polymer percentage [
8]. As shown in
Table 4 in the DMF/acetone (FA 6-4 Px, x = 6, 8, 10%) dope solutions, the viscosity increased with increasing polymer content to be 70, 175, and 405 cp, respectively, but it decreased when fixing polymer percentage and increasing the weight fraction of acetone. Overall, in this study, the suitable viscosity for electrospinning was found in a range between 70 and 400 cp for all DMF/acetone dope solutions. Viscosity and surface tension values were found in the appropriate range for electrospinning that was reported in the literature [
41,
42].
3.2. Membrane Surface Characterizations
Concerning the structural properties of the membrane, the thickness, porosity, pore size, and contact angle were investigated to ensure that the membranes were suitable for water treatment applications.
Table 5 shows the thickness and pore size results of PVDF-HFP nanofiber membranes. The polymer percentage used in the preparation of the nanofiber membranes affected the thickness of these membranes. For example, the FA 6-4 P6 membrane had a thickness of 64.8 µm, which increased as the polymer percentage increased from 8% to 10%. The FA 6-4 P6 and FA 6-4 P8 membranes had a thickness of 184 and 251 µm, respectively. Furthermore, the difference in thickness of these three membranes was a little small between the FA 6-4 P6 and FA 6-4 P8 membranes, but it was large between those two membranes and the FA 6-4 P10 membrane, which may have resulted from the difference in fiber collection time during electrospinning. The electrospinning of the FA 6-4 P10 membrane took longer than the other two membranes. In the case of fixing the polymer percentage and increasing the acetone weight fraction, the membrane became thinner. The thickness of the FA 5-5 P10 and FA 4-6 P10 membranes sharply decreased to 103 and 77.5 µm, respectively, compared with the FA 6-4 P10 membrane. In addition, the NIPS membrane was the thinnest one compared to all nanofiber membranes. In the same way, the increase in acetone weight fraction affected the mean pore size by making it narrower. The FA 4-6 P10 membrane with the highest amount of acetone possessed a mean pore size that was smaller than the FA 5-5 P10 and FA 6-4 P10 membranes. The polymer percentage had little effect on the mean pore size, but all membranes, in this case, recorded mean pore sizes in the same range between 202 and 218. However, the mean pore sizes of all the prepared membranes were found to be in a good range—lower than 0.3 µm (300 nm). The results showed that the thickness of the membrane is directly proportional to the viscosity of the dope solution. So, with the increase in the viscosity of dope solutions, the thickness of the membranes increases and vice versa. In addition, especially when the polymer percentage is fixed, a thinner nanofiber membrane tends to form a smaller pore size [
43].
The porosity of electrospun PVDF-HFP membranes was tested in kerosene and the results are shown in
Figure 3. Firstly, the porosity of nanofiber membranes increased as the PVDF-HFP percentage increased, the highest of which was recorded for the FA 6-4 P10 membrane. The porosity of FA 6-4 P10 was 73.07%, while the porosities of the FA 6-4 P8 membrane and FA 6-4 P6 membrane were 68.46.16% and 60%, respectively. In the case of fixed polymer percentage, the porosities of three membranes (FA 6-4 P10, FA 5-5 P10, and FA 4-6 P10) were in the same range and were not largely affected by the increasing acetone concentration. Unlike water uptake, the water uptake of the nanofiber membranes increased with the increase in acetone weight fraction. The lowest percentage of water uptake was 33.3%, which was recorded by FA 6-4 P10. The water uptake increased to 60 and 75% when the acetone weight fraction was increased in the FA 5-5 P10 and FA 4-6 P10 membranes, respectively. Generally, all nanofiber membranes possess excellent porosity greater than 60%.
The hydrophobicity of the membranes was also tested by measuring the water contact angle for each face of the membrane. The results showed that the back face of almost all the membranes had a higher hydrophobicity property. As shown in
Figure 4, the highest contact angle was recorded by DMF/acetone membranes in two ratios of 5:5 and 4:6 (FA 5-5 P10 and FA 4-6 P10) on both sides, which were (133.22° and 138.39°) and (122.98° and 133.66°) for the front and back faces, respectively. Among membranes containing 10% of PVDF, the lowest contact angle was recorded by the one with a weight ratio of 6:4 which was 116.56° for the front face and 114.95° for the back face. When the solvent weight ratio of DMF/acetone was fixed at 6:4 with a changing PVDF-HFP percentage (FA 6-4 P6, FA 6-4 P8, and FA 6-4 P10), the back face of all membranes showed a superior water contact angle compared with the front face. According to the back face contact angle of DMF/acetone membranes, the best percentage of PVDF-HFP is 8% since its contact angle was 132.82°, whereas in the FA P6 and FA P10 membranes, it was 122.75° and 114.95° for the back face, respectively. On the other hand, the front faces of the FA 6-4 P6 and FA 6-4 P10 membranes had almost the same contact angle of 116.65° and 116.56°, respectively. However, the front face contact angle of the FA 6-4 P8 membrane was significantly lower than the back face. Nonetheless, all membranes were sufficiently hydrophobic as their water contact angles were greater than 90° [
44].
As shown in the literature, the LEP value of the membrane is directly influenced by the membrane thickness and pore size, and this explains the fluctuation of LEP values. A high LEP value refers to a low pore size and the optimum thickness of the membrane.
Figure 5 shows membrane LEP values. The membranes with different polymer weights (FA 6-4 P6, FA 6-4 P8, and FA 6-4 P10) showed that membrane with the lowest polymer percentage (FA 6-4 P6) had the highest LEP, which resulted from its lower pore size. The LEP value increased from 0.7 to 0.9 bar with the polymer percentage increasing from 8% in the FA 6-4 P8 membrane to 10% in the FA 6-4 P10 membrane, as a result of a decrease in pore size and increased thickness. In the case of an equal weight fraction of DMF and acetone, the LEP value remained at 0.9 bar for the FA 6-4 P10 membrane, even though the FA 6-4 P10 membrane had a much higher thickness compared to the FA 5-5 P10 membrane, and this difference may have disappeared because the FA 6-4 P10 membrane possessed a larger pore size than the FA 5-5 P10 membrane, which accelerated the wettability and reduced the LEP value. In contrast, when the weight fraction of acetone was higher than that of DMF (FA 4-6 P10), the LEP value reduced to 0.5 bar, since the thickness of the membrane significantly decreased but the pore size slightly decreased [
32].
The crystalline phase composition and thermal properties of pure PVDF-HFP and nanofiber membranes were investigated using the DSC technique. The DSC thermograms of PVDF-HFP and the nanofiber membranes are presented in
Figure 6. It should be noted that the glass transition temperature (Tg) of PVDF-HFP is around −35 °C, as mentioned in the literature [
45]. From the DSC curves, thermodynamic parameters were calculated and listed in
Table 6. As shown in
Table 6, the increase in PVDF-HFP content led to an increase in melting enthalpy and crystallinity percentage, which was observed in three membranes of FA 6-4 Px (x = 6, 8, and 10). FA 6-4 P6 had a very low melting enthalpy and crystallinity percentage compared to FA 6-4 P8 and FA 6-4 P10. The values of melting enthalpy and crystallinity of membranes containing 8 and 10% were very close. Thus, in membranes produced with a high polymer percentage, the crystalline region was higher than the amorphous region. Overall, the crystallinity of all nanofiber membranes was in the very low range and lower than the crystal content of pure PVDF-HFP.
The pure PVDF-HFP curve, as shown in
Figure 6, has two peaks appearing at 119 and 135 °C, which relate to the α-phase and β-phase, respectively. However, the nanofiber membrane curves have only one peak, with the other appearing as a broad low peak or broad drop in the curve line. An endothermic peak appeared between 130 and 148 °C in different nanofiber membranes due to PVDF-HFP copolymer melting. In addition, compared to the curve of pure PVDF-HFP, it is also related to the presence of the β-phase, whereas the disappearance of the first peak (at 119 °C) resulted from the absence of the α-form. The broad drop in the curve line of nanofiber membranes has been considered a second T
g (upper T
g), and it may indicate reorganization inside α-crystals, the melting of paracrystalline domains, or molecular motions corresponding to an α-relaxation in the crystalline/amorphous interface [
46]. All these explanations make sense with the disappearance of the α-phase peak. Some studies also reported that the first curve that appeared in the range of 50 to 125 °C in different nanofiber membranes was considered a peak, and it refers to the presence of residual moisture [
47,
48]. The other suggested that the double peak of melting is attributed to its polymorphic structure; it was also referring to the presence of recrystallization of molten polymer and imperfect crystals in pure PVDF-HFP. Furthermore, it may be related to the difference in the arrangement of bonding such as “head-to-head” or “tail-to-tail” in PVDF-HFP membranes, which affects their thermodynamic behavior and crystalline phase formation [
49,
50]. Further research revealed that this curve is not considered an individual peak but rather a drop caused by phase transition [
51]. Overall, the DSC result concluded that the amorphous phase dominates in all nanofiber membranes and the crystallinity is very low.
The FTIR results of different nanofibers are presented in
Figure 7. FTIR peaks give information about the functional groups and crystalline phases that exist in membranes. Thus, the vibrational peak at 471 cm
−1 is attributed to C–F wagging vibrations. The bands observed at 839 cm
−1 and 795 cm
−1 are assigned to CH
2 rocking vibrations, while its swinging vibration was shown at 1179 cm
−1. The vibrational peaks at 509 and 1279 cm
−1 are assigned to the bending and asymmetric stretching vibrations of the CF
2 group. The peak observed at 1399 cm
−1 is related to the wagging vibration of CH
2 or stretching vibrations of the CF group. All these vibrational peaks were found in the FTIR spectra of pure PVDF-HFP and all nanofiber membranes. Besides that, the crystallinity of pure PVDF-HFP and all nanofiber membranes was illustrated by FTIR and XRD peaks. The crystalline content of PVDF is less than 60%, as mentioned in the literature. Nonetheless, the amorphous part is not well studied and no trusted information about how it affects the XRD and FITR spectra can be found. So, this part focused on describing the crystalline phases of pure PVDF-HFP and its nanofiber membranes. The peaks of FTIR and XRD spectra describe the nonpolar crystalline (i.e., α-phase) and polar β- and γ-phases. The nonpolar crystalline α-phase of pure PVDF-HFP was observed at 1064, 795, 760, 612, 509, and 485 cm
−1, while the peaks at 1300, 1279, and 839 cm
−1 were assigned to amorphous β-phase and 1179 cm
−1 for the γ-phase. The dual character peaks that appeared at 975 were due to a mixture of α- and γ-phases, while 871 cm
−1 was attributed to a mixture of β- and γ-phases [
23,
52,
53,
54]. As shown in
Figure 7, some peaks of nonpolar crystalline α-phase disappeared in the spectra of membranes which were exhibited in pure PVDF-HFP spectra; but still, the other peaks of α-phase appeared, although with lower intensity (i.e., 485, 509, 612, and 1064 cm
−1). Furthermore, all the vibrational peaks of the amorphous polar β-phase and semipolar γ-phase appeared more intensely and broadly compared to the same peaks in the pure PVDF-HPF spectra. However, these results reveal that all membranes contain an amorphous polar β-phase and semipolar γ-phase and a very slight amount of nonpolar α-phase. Changing the solvent weight ratio in DMF/acetone binary solvents could influence the volatility of the solvent system, consequently affecting the evaporation rate during electrospinning. The vapor pressure of each solvent system indicates the evaporation rate, which increased as the acetone weight ratio increased. In summary, as the volatility, vapor pressure, evaporation rate, or voltage are increased during electrospinning, so does the formation of the β-phase in the produced membrane [
55,
56].
The XRD spectra of pure PVDF-HFP and nanofiber membranes provided good agreement with the FTIR results, where XRD spectra revealed peaks corresponding to a crystalline nonpolar α-phase and amorphous polar β- and γ-phases, as FTIR spectra had previously revealed. The XRD spectra of nanofiber membranes are presented in
Figure 8. The strong peaks at 17.6, 18.4, and 19.9 correspond to 100, 020, and 110, respectively, which are related to the nonpolar α-phase. This finding demonstrates that the α-phase exists as the primary phase in powder PVDF-HFP. This does not mean that the PVDF-HFP contains only an α-phase, but also contains the γ- and β-phases, which appeared in weak peaks at 20.3 and 20.9, corresponding to 101 and 110/200, respectively. All PVDF-HFP electrospun nanofiber membranes showed peaks of the α-phase and amorphous polar β- and γ-phases [
24,
57]. Finally, DSC and FTIR confirmed XRD results; all nanofiber membranes exhibited a semicrystalline nature.
3.3. Morphological Studies
To evaluate the morphological structure of PVDF-HFP nanofiber membranes, the AFM technique was used. It was used to examine the internal construction of polymeric nanofibers and determine the average fiber diameter (AFD). From the 3D AFM images (
Figure 9), the average surface roughness (R
a) and root mean square roughness (R
q) were calculated for PVDF-HFP nanofiber membranes and are listed in
Table 7. AFM found that the average surface roughness of the nanofiber membranes increased with polymer percentage. The higher average surface roughness was recorded by the membrane produced with the highest weight ratio of acetone (FA 4-6 P10), which agreed with its contact angle (133.66°). When the weight ratio of DMF and acetone was equal, the average surface roughness sharply decreased to 258.1 nm. In the same way, the root mean square roughness was 697.8 nm for the FA 6-4 P10 membrane, which was the highest value. Since the FA 6-4 Px (x = 6 and 8) polymeric solution had a high surface tension of 38.5 and 39.52 mNm
−1, it formed many beads in the fibers. Because the surface tension of the polymeric solution in the FA 6-4 P10 membrane was lower, this effect vanished. Thus, a lower polymer concentration always increases the bead formation and produces nonuniform fibers. This can be concluded by the fact that when the surface tension is lower or equal to 37 mNm
−1, such as in the case of the FA 6-4 P10, FA 5-5 P10, and FA 4-6 P10 polymeric solutions, smooth and uniform nanofibers will be produced. When the surface tension is above 37 mNm
−1, beads start to form on nonuniform fibers and nanofiber networks that are heterogeneous, as shown in
Figure 9a–c. In addition, polymer concentration is an essential parameter that affects the polymeric solution’s way of spinning in the electrospinning process and, consequently, the morphology of fibers in electrospun membranes.
The FA 6-4 P6 membrane exhibited heterogeneous networks with nonuniform beaded nanofibers, while the nanofibers improved in the 6-4 P8 membrane, where the fiber was more regular and the formation of the beads on the fibers was decreased. However, the nanofiber networks were almost organized (homogeneous), with smoother fibers in the membrane produced with a high polymer percent of FA 6-4 P10 (high viscosity). Polymeric solutions with a high polymer percentage had a good viscoelastic force that made the jet during electrospinning continuously elongate due to it fitting the coulombic and electrostatic forces, whereas the jet of the polymeric solution with a low polymer percentage underwent partial fracture because its viscoelastic force was low and could not fit the other forces that affect the electrospinning operation [
29,
41]. On the other hand, the beads did not form on the nanofibers of the FA 5-5 P10 and FA 4-6 P10 membranes, as observed in
Figure 9d,e, and the nanofibers were perfectly entangled. The viscosities of the polymeric solutions (375 and 339.5 cp for FA 5-5 P10 and FA 4-6 P10) are attributed to this effect because they are higher than the viscosities of AF 6-4 P6 and FA 6-4 P8, which have viscosities of 70 and 175 cp and do not have these features. The second thing that may be responsible for this behavior is the solvent evaporation rate during the electrospinning process which is influenced by the vapor pressure and volatility property of the solvent. DMF has a low vapor pressure and volatility, whereas acetone has a high vapor pressure and volatility; therefore, different solvents’ mixing composition resulted in different volatility and vapor pressure. In addition, the smaller fiber diameter produced by the solution possesses low viscosity and low vapor pressure (high boiling point) [
58]. The requirement for low viscosity is to produce fine fibers to prevent polymer macromolecules from becoming entangled, as occurs in solutions with high viscosity [
41]. In addition, the morphology of the NIPS membrane in
Figure 9f differs from all other membranes (
Figure 9a–e). The NIPS membrane shows a spongy texture, while the electrospun membranes show nanofibers. The roughness of the NIPS membrane was sharply decreased to be at the lowest level compared to all electrospun nanofiber membranes, and the lowest difference in Ra values between them was 144 nm. These observations explain the previous contact angle results, which showed that the contact angle of the NIPS membrane was much lower than the contact angle of all electrospun nanofiber membranes.
3.4. DCMD Application
MD tests were used to evaluate the performance of different PVDF-HFP nanofiber membranes. The feed side used brine water at 70 °C, and the permeate temperature was 20 °C. Water vapor passed through the pores of the membranes due to the difference in vapor pressure between the two sides. The NIPS membrane did not give any flux of water under these experimental conditions because it had a very small pore size and very low porosity.
Table 8 shows the average water flux and salt rejection of different nanofiber membranes. Since the FA 6-4 P6 membrane exhibited a larger pore size and higher porosity than the FA 6-4 P8 and FA 6-4 P10 membranes, it also exhibited good permeability in the MD test.
As can be seen in
Figure 10, the flux of water was maintained above 25 kg m
−2 h
−1 during the two-hour test by three different membranes: FA 6-4 P6, FA 5-5 P10, and FA 4-6 P10. They achieved a higher water flux compared to the PVDF membrane filter disc. According to the average water flux listed in
Table 8, the PVDF membrane filter disc provided 22 kg m
−2 h
−1, while FA 6-4 P6, FA 5-5 P10, and FA 4-6 P10 were all greater than 38 kg m
−2 h
−1. The other two membranes, FA 6-4 P8 and FA 6-4 P10, gave average water flux values very close to those of the PVDF membrane filter disc and showed almost the same behavior. The behavior of the FA 6-4 Px (x = 6, 8, and 10) membranes in the MD test shows that as the PVDF-HFP content increased, the water flux decreased. This result is expected due to the hydrophobicity property of PVDF-HFP, which increased as the polymer content rose. The roughness of membranes also increased as the polymer content increased, which made the membranes have high hydrophobicity and made it difficult for vaporized water to pass across the membrane pores. Since the porosity is directly proportional to the permeability of the membrane, this provided a good argument for these results. As mentioned previously, the porosities of the FA 6-4 Px (x = 6, 8, and 10) membranes were 81.27, 79.16, and 63.45%, respectively; consequently, the permeability was found to be higher in the FA 6-4 P6 membrane than in the FA 6-4 P8 membrane and at a lower level in the FA 6-4 P10 membrane. The permeability behavior of the FA 6-4 Px (x = 6, 8, and 10) membranes in the first 50 min was constant; after that, it slightly decreased, except for the FA 6-4 P10 membrane. On the other hand, even though the porosity of the FA 6-4 P10 membrane was higher than the porosities of the FA 5-5 P10 and FA 4-6 P10 membranes by 5%, the permeability of the FA 5-5 P10 and FA 4-6 P10 membranes increased by approximately 20 kg m
−2 h
−1 compared to the permeability of the FA 6-4 P10 membrane.
The average salt rejection of PVDF-HFP nanofiber membranes was found to be above 90%, as shown in
Table 8, which proves the efficiency of these nanofiber membranes in water treatment and contamination removal. The FA 5-5-P10 membrane achieved the perfect performance in terms of permeability and salt rejection. Even though a lower water flux in the MD test was achieved with the FA 6-4 P8 and FA 6-4 P10 membranes, they achieved a higher salt rejection percentage (99.9%). The FA 6-4 P6 and FA 4-6 P10 membranes had a higher water flux but slightly lower salt rejection (91.5% and 91.7%, respectively) compared to the others. Regardless, during the MD experiments, all PVDF-HFP electrospun nanofiber membranes demonstrated good permeability and salt rejection for two hours with no damaged or sharply increased conductivity.