Insulin-Loaded Soybean Trypsin Inhibitor-Chitosan Nanoparticles: Preparation, Characterization, and Protective Effect Evaluation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Feedstocks and Reagents
2.2. STI Preparation [28]
2.3. Preparation of Insulin-Loaded Soybean Trypsin Inhibitor-Chitosan Complex Coacervation [29,30]
2.4. Determination of Insulin by RP-HPLC
2.5. Calculation of Encapsulation Efficiency
2.6. Effects of CS, STI, and pH on Insulin Encapsulation Efficiency and Particle Characteristics
2.6.1. Optimization of CS Concentration
2.6.2. Optimization of STI Concentration
2.6.3. Optimization of pH of the Complexation in the Mixing System
2.7. Characterization of INs-STI-CS Nanoparticles
2.7.1. Particle Size and PDI Value
2.7.2. Microscopic Morphological Characteristics
2.8. Nanoparticles In Vitro Simulated Gastrointestinal Sustained Release Insulin [2]
2.9. Structural Stability of Insulin Released from the Nanoparticles
2.10. In Vitro Gastrointestinal Digestion of INs-STI-CS Nanoparticles
2.10.1. Simulated Gastric Digestion
2.10.2. Simulated Intestinal Digestion
2.11. Statistical Analysis
3. Results and Discussion
3.1. Effects of Different Factors on Insulin Encapsulation Efficiency, Particle Size, and PDI Value
3.2. Microstructure of Complex Coacervates Nanoparticles
3.2.1. Scanning Electron Microscopy (SEM)
3.2.2. Transmission Electron Microscopy (TEM)
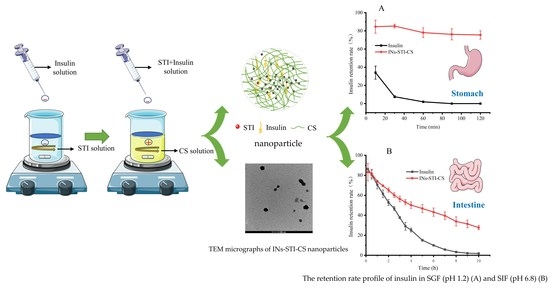
3.3. In Vitro Simulated Gastrointestinal Slow Release
3.4. Stability of Insulin Extracted from Nanoparticles
3.5. In Vitro Simulation of Gastrointestinal Digestion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Phan, V.H.G.; Mathiyalagan, R.; Nguyen, M.T.; Tran, T.T.; Murugesan, M.; Ho, T.N.; Huong, H.; Yang, D.C.; Li, Y.; Thambi, T. Ionically cross-linked alginate-chitosan core-shell hydrogel beads for oral delivery of insulin. Int. J. Biol. Macromol. 2022, 222, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Sarhadi, S.; Moosavian, S.A.; Mashreghi, M.; Rahiman, N.; Golmohamadzadeh, S.; Tafaghodi, M.; Sadri, K.; Chamani, J.; Jaafari, M.R. B12-functionalized PEGylated liposomes for the oral delivery of insulin: In vitro and in vivo studies. J. Drug Deliv. Sci. Technol. 2022, 69, 103141. [Google Scholar] [CrossRef]
- Xiang, Z.; Xie, H.; Tong, Q.; Pan, J.; Wan, L.; Fang, J.; Chen, J. Revealing hypoglycemic and hypolipidemic mechanism of Xiaokeyinshui extract combination on streptozotocin-induced diabetic mice in high sucrose/high fat diet by metabolomics and lipidomics. Biomed. Pharmacother. 2021, 135, 111219. [Google Scholar] [CrossRef]
- Xu, M.; Huang, J.; Jiang, S.; He, J.; Wang, Z.; Qin, H.; Guan, Y.Q. Glucose sensitive konjac glucomannan/concanavalin A nanoparticles as oral insulin delivery system. Int. J. Biol. Macromol. 2022, 202, 296–308. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Chen, L.; Duan, X.; Li, X.; Ren, H. Microparticles based on alginate/chitosan/casein three-dimensional system for oral insulin delivery. Polym. Adv. Technol. 2021, 32, 4352–4361. [Google Scholar] [CrossRef]
- Ramesan, R.M.; Sharma, C.P. Challenges and advances in nanoparticle-based oral insulin delivery. Expert Rev. Med. Devices 2009, 6, 665–676. [Google Scholar] [CrossRef] [PubMed]
- Jamshidi, M.; Ziamajidi, N.; Abbasalipourkabir, R.; Khodadadi, I.; Moradi, M.; Dehghan, A.; Kalantarian, G. Study the effect of insulin-loaded trimethylchitosan nanoparticles on HepG2 cell line. Gene Rep. 2022, 27, 101602. [Google Scholar] [CrossRef]
- Mumuni, M.A.; Kenechukwu, F.C.; Ofokansi, K.C.; Attama, A.A.; Diaz, D.D. Insulin-loaded mucoadhesive nanoparticles based on mucin-chitosan complexes for oral delivery and diabetes treatment. Carbohydr. Polym. 2020, 229, 115506. [Google Scholar] [CrossRef]
- Chen, T.; Li, S.; Zhu, W.; Liang, Z.; Zeng, Q. Self-assembly pH-sensitive chitosan/alginate coated polyelectrolyte complexes for oral delivery of insulin. J. Microencapsul. 2019, 36, 96–107. [Google Scholar] [CrossRef]
- Zhang, Y.W.; Tu, L.L.; Tang, Z.; Wang, Q.; Zheng, G.L.; Yin, L.N. pH-sensitive chitosan-deoxycholic acid/alginate nanoparticles for oral insulin delivery. Pharm. Dev. Technol. 2021, 26, 943–952. [Google Scholar] [CrossRef]
- Kedir, W.M.; Abdi, G.F.; Goro, M.M.; Tolesa, L.D. Pharmaceutical and drug delivery applications of chitosan biopolymer and its modified nanocomposite: A review. Heliyon 2022, 8, e10196. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, F.C.; Silva, M.C.D.; Silva, H.N.D.; Albuquerque, D.; Gomes, A.A.R.; Silva, S.M.L.; Fook, M.V.L. Progress in the Development of Chitosan Based Insulin Delivery Systems: A Systematic Literature Review. Polymers 2020, 12, 2499. [Google Scholar] [CrossRef]
- Huang, M.; Xu, Y.; Xu, L.; Bai, Y.; Xu, X. Interactions of water-soluble myofibrillar protein with chitosan: Phase behavior, microstructure and rheological properties. Innov. Food Sci. Emerg. Technol. 2022, 78, 103013. [Google Scholar] [CrossRef]
- Lin, Y.-H.; Mi, F.-L.; Chen, C.-T.; Chang, W.-C.; Peng, S.-F.; Liang, H.-F.; Sung, H.-W. Preparation and Characterization of Nanoparticles Shelled with Chitosan for Oral Insulin Delivery. Macromolecules 2007, 8, 146–152. [Google Scholar] [CrossRef]
- Mukhopadhyay, P.; Sarkar, K.; Chakraborty, M.; Bhattacharya, S.; Mishra, R.; Kundu, P.P. Oral insulin delivery by self-assembled chitosan nanoparticles: In vitro and in vivo studies in diabetic animal model. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 376–382. [Google Scholar] [CrossRef]
- Wu, Y.; Li, W.; Colombo, E.; Martin, G.J.O.; Ashokkumar, M. Kinetic and mechanistic study of ultrasonic inactivation of Kunitz (KTI) and Bowman-Birk (BBI) inhibitors in relation to process-relevant parameters. Food Chem. 2023, 401, 134129. [Google Scholar] [CrossRef]
- Li, X.; Dong, D.; Hua, Y.; Chen, Y.; Kong, X.; Zhang, C. Soybean whey protein/chitosan complex behavior and selective recovery of kunitz trypsin inhibitor. J. Agric. Food Chem. 2014, 62, 7279–7286. [Google Scholar] [CrossRef]
- Karlund, A.; Paukkonen, I.; Gomez-Gallego, C.; Kolehmainen, M. Intestinal Exposure to Food-Derived Protease Inhibitors: Digestion Physiology- and Gut Health-Related Effects. Healthcare 2021, 9, 1002. [Google Scholar] [CrossRef] [PubMed]
- Roychaudhuri, R.; Sarath, G.; Zeece, M.; Markwell, J. Reversible denaturation of the soybean Kunitz trypsin inhibitor. Arch. Biochem. Biophys. 2003, 412, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Y.; Ying, Z.; Li, W.; Li, H.; Liu, X. Trypsin Inhibitor from Soybean Whey Wastewater: Isolation, Purification and Stability. Appl. Sci. 2022, 12, 10084. [Google Scholar] [CrossRef]
- Kennedy, A.R. The Bowman-Birk inhibitor from soybeans as an anticarcinogenic agent. Am. J. Clin. Nutr. 1998, 68, 1406S–1412S. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Wang, D.-F.; Li, H.-Y.; Xu, Y.; Zhang, L. Preparation and properties of chitosan-soybean trypsin inhibitor blend film with anti-Aspergillus flavus activity. Ind. Crops Prod. 2009, 29, 541–548. [Google Scholar] [CrossRef]
- Krishnan, H.B.; Wang, T.T. An effective and simple procedure to isolate abundant quantities of biologically active chemopreventive Lunasin Protease Inhibitor Concentrate (LPIC) from soybean. Food Chem. 2015, 177, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Nieto-Veloza, A.; Wang, Z.; Zhong, Q.; D’Souza, D.; Krishnan, H.B.; Dia, V.P. Lunasin protease inhibitor concentrate decreases pro-inflammatory cytokines and improves histopathological markers in dextran sodium sulfate-induced ulcerative colitis. Food Sci. Hum. Wellness 2022, 11, 1508–1514. [Google Scholar] [CrossRef]
- Li, Q.; Huang, L.; Luo, Z.; Tamer, T.M. Stability of trypsin inhibitor isolated from potato fruit juice against pH and heating treatment and in vitro gastrointestinal digestion. Food Chem. 2020, 328, 127152. [Google Scholar] [CrossRef]
- Burshtein, G.; Itin, C.; Tang, J.C.Y.; Galitzer, H.; Fraser, W.D.; Schwartz, P. The combined effect of permeation enhancement and proteolysis inhibition on the systemic exposure of orally administrated peptides: Salcaprozate sodium, soybean trypsin inhibitor, and teriparatide study in pigs. Int. J. Pharm. X 2021, 3, 100097. [Google Scholar] [CrossRef]
- Chuang, E.Y.; Lin, K.J.; Su, F.Y.; Chen, H.L.; Maiti, B.; Ho, Y.C.; Yen, T.C.; Panda, N.; Sung, H.W. Calcium depletion-mediated protease inhibition and apical-junctional-complex disassembly via an EGTA-conjugated carrier for oral insulin delivery. J. Control. Release 2013, 169, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, R.; Li, H.; Li, Y.; Liu, X. Interactions between Soybean Trypsin Inhibitor and Chitosan in an Aqueous Solution. Polymers 2023, 15, 1594. [Google Scholar] [CrossRef]
- Liu, C.; Kou, Y.; Zhang, X.; Dong, W.; Cheng, H.; Mao, S. Enhanced oral insulin delivery via surface hydrophilic modification of chitosan copolymer based self-assembly polyelectrolyte nanocomplex. Int. J. Pharm. 2019, 554, 36–47. [Google Scholar] [CrossRef]
- Ji, N.; Hong, Y.; Gu, Z.; Cheng, L.; Li, Z.; Li, C. Preparation and Characterization of Insulin-Loaded Zein/Carboxymethylated Short-Chain Amylose Complex Nanoparticles. J. Agric. Food Chem. 2018, 66, 9335–9343. [Google Scholar] [CrossRef]
- De Marchi, J.G.B.; Ce, R.; Onzi, G.; Alves, A.C.S.; Santarem, N.; Cordeiro da Silva, A.; Pohlmann, A.R.; Guterres, S.S.; Ribeiro, A.J. IgG functionalized polymeric nanoparticles for oral insulin administration. Int. J. Pharm. 2022, 622, 121829. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Santos, J.L.; Tian, H.; Huang, H.; Hu, Y.; Liu, L.; Leong, K.W.; Chen, Y.; Mao, H.Q. Scalable fabrication of size-controlled chitosan nanoparticles for oral delivery of insulin. Biomaterials 2017, 130, 28–41. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, J.R.; Yu, F.; Venkatasubramanian, R.; Nielsen, L.H.; Nielsen, H.M.; Boisen, A.; Rades, T.; Mullertz, A. In Vitro, Ex Vivo and In Vivo Evaluation of Microcontainers for Oral Delivery of Insulin. Pharmaceutics 2020, 12, 48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Remawi, M.; Jaber, N.; Elsayed, A.; Alsafadi, D.; Salah, K.A. Stabilization of insulin using low molecular weight chitosan carbonate nanocarrier. Carbohydr. Polym. 2022, 291, 119579. [Google Scholar] [CrossRef] [PubMed]
- Abdelwahed, W.; Degobert, G.; Stainmesse, S.; Fessi, H. Freeze-drying of nanoparticles: Formulation, process and storage considerations. Adv. Drug. Deliv. Rev. 2006, 58, 1688–1713. [Google Scholar] [CrossRef]
- Sahoo, P.; Leong, K.H.; Nyamathulla, S.; Onuki, Y.; Takayama, K.; Chung, L.Y. Optimization of pH-responsive carboxymethylated iota -carrageenan/chitosan nanoparticles for oral insulin delivery using response surface methodology. React. Funct. Polym. 2017, 119, 145–155. [Google Scholar] [CrossRef]
- Hadiya, S.; Radwan, R.; Zakaria, M.; El-Sherif, T.; Hamad, M.A.; Elsabahy, M. Nanoparticles integrating natural and synthetic polymers for in vivo insulin delivery. Pharm. Dev. Technol. 2021, 26, 30–40. [Google Scholar] [CrossRef]
- Litwack, G. Pancreatic hormones: Insulin and glucagon. Hormones 2022, 6, 123–157. [Google Scholar] [CrossRef]
- Jones, C. Circular dichroism of biopharmaceutical proteins in a quality-regulated environment. J. Pharm. Biomed. Anal. 2022, 219, 114945. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, Y.; Zhao, D. Structural analysis of biomacromolecules using circular dichroism spectroscopy. In Advanced Spectroscopic Methods to Study Biomolecular Structure and Dynamics; Academic Press: Cambridge, MA, USA, 2023; pp. 77–103. [Google Scholar] [CrossRef]
- Zhang, F.; Pei, X.; Peng, X.; Gou, D.; Fan, X.; Zheng, X.; Song, C.; Zhou, Y.; Cui, S. Dual crosslinking of folic acid-modified pectin nanoparticles for enhanced oral insulin delivery. Biomater. Adv. 2022, 135, 212746. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Liu, R.; Feng, Q.; Li, H.; Li, Y.; Liu, X. Insulin-Loaded Soybean Trypsin Inhibitor-Chitosan Nanoparticles: Preparation, Characterization, and Protective Effect Evaluation. Polymers 2023, 15, 2648. https://doi.org/10.3390/polym15122648
Zhang Y, Liu R, Feng Q, Li H, Li Y, Liu X. Insulin-Loaded Soybean Trypsin Inhibitor-Chitosan Nanoparticles: Preparation, Characterization, and Protective Effect Evaluation. Polymers. 2023; 15(12):2648. https://doi.org/10.3390/polym15122648
Chicago/Turabian StyleZhang, Yihao, Ruijia Liu, Qixu Feng, He Li, You Li, and Xinqi Liu. 2023. "Insulin-Loaded Soybean Trypsin Inhibitor-Chitosan Nanoparticles: Preparation, Characterization, and Protective Effect Evaluation" Polymers 15, no. 12: 2648. https://doi.org/10.3390/polym15122648