Optimized End Functionality of Silane-Terminated Liquid Butadiene Rubber for Silica-Filled Rubber Compounds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of the Functional Initiator
2.1.1. Synthesis of (E)-4,4′-(diazene-1,2-diyl)bis-(4-cyano-N-(3-(triethoxysilyl)propyl)pentanamide) (difunctional initiator)
2.1.2. Synthesis of (E)-4,4′-(diazene-1,2-diyl)bis(4-cyano-N,N-bis(3-(triethoxysilyl)propyl)pentanamide) (tetrafunctional initiator)
2.1.3. Polymerization
2.1.4. Compounding
2.2. Characterization
2.2.1. Gel Permeation Chromatography (GPC)
2.2.2. Proton Nuclear Magnetic Resonance Spectroscopy (1H NMR)
2.2.3. Differential Scanning Calorimetry (DSC)
2.2.4. Payne Effect
2.2.5. Mooney Viscosity
2.2.6. Curing Characteristics
2.2.7. Solvent Extraction and Crosslink Density
2.2.8. Mechanical Properties
2.2.9. Abrasion Resistance
2.2.10. Dynamic Viscoelastic Properties
2.3. Synthesis of the Functionalized Initiator
2.3.1. Di-functional Initiator (2-Azo-initiator)
2.3.2. Tetra-Functional Initiator (4-Azo-initiator)
2.4. Synthesis of Functionalized LqBR
Radical Polymerization
2.5. Manufacture of Rubber/Silica Compounds and Vulcanizates
3. Results and Discussion
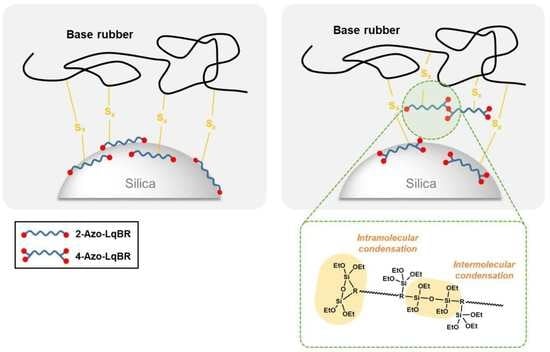
3.1. Synthesis of Functional Initiators
3.2. Synthesis of F-LqBRs
3.3. Payne Effect
3.4. Cure Characteristics and Mooney Viscosity of the Compounds
3.5. Solvent Extraction and Crosslink Density
3.6. Mechanical Properties and DIN Abrasion Loss
3.7. Dynamic Viscoelastic Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sadeghian, S.; Wintersberger, P.; Laschke, M.; Hassenzahl, M. Designing Sustainable Mobility: Understanding Users’ Behavior. In Proceedings of the 14th International Conference on Automotive User Interfaces and Interactive Vehicular Applications, Ingolstadt, Germany, 17–20 September 2022; pp. 34–44. [Google Scholar]
- Köhler, J.; Whitmarsh, L.; Nykvist, B.; Schilperoord, M.; Bergman, N.; Haxeltine, A. A transitions model for sustainable mobility. Ecol. Econ. 2009, 68, 2985–2995. [Google Scholar] [CrossRef]
- Geronikolos, I.; Potoglou, D. An exploration of electric-car mobility in Greece: A stakeholders’ perspective. Case Studies Trans. Pol. 2021, 9, 906–912. [Google Scholar] [CrossRef]
- Kley, F.; Lerch, C.; Dallinger, D. New business models for electric cars—A holistic approach. Energy Policy 2011, 39, 3392–3403. [Google Scholar] [CrossRef] [Green Version]
- Whitby, R.D. Electric cars and winter tires. Tribol. Lubr. Technol. 2022, 78, 80. [Google Scholar]
- Mirzanamadi, R.; Gustafsson, M. Users’ Experiences of Tyre Wear on Electric Vehicles: A Survey and Interview Study; Statens väg-och transportforskningsinstitut: Linköping, Sweden, 2022. [Google Scholar]
- Chan, C.C. An overview of electric vehicle technology. Proc. IEEE 1993, 81, 1202–1213. [Google Scholar] [CrossRef]
- Paik, H.J.; Kim, W.; Yeom, G.; Kim, D.; Choi, H.; Song, S. Rubber Composition for Manufacturing Tires Comprising Terminally Modified Liquid Polybutadienes. K.R. Patent 102022-0032425, 16 March 2022. [Google Scholar]
- Han, S.C.; Choe, S.J.; Han, M.H. Compounding technology of silica-filled rubber. Rubber Technol. 2001, 2, 100–116. [Google Scholar]
- Lee, J.W.; Chung, C.B.; Choi, I.C. Severity factors affecting tire wear. Polymer 2005, 29, 48–53. [Google Scholar]
- Malas, A.; Pal, P.; Das, C.K. Effect of expanded graphite and modified graphite flakes on the physical and thermo-mechanical properties of styrene butadiene rubber/polybutadiene rubber (SBR/BR) blends. Mat. Des. 2014, 55, 664–673. [Google Scholar] [CrossRef]
- Waddell, B.W.; Lee, S.; Lin, T.Y.; Yang, E. Factors influencing silica’s effectiveness in PCR tires. Rubber Plast. News 2019, 16–19. [Google Scholar]
- Cichomski, E.M. Silica-Silane Reinforced Passenger Car Tire Treads. Ph.D. Thesis, University of Twente, Enschede, The Netherlands, 2015. [Google Scholar]
- Ahmad, S.; Schaefer, R.J. The B. F. Goodrich Company. U.S. Patent 4,519,430, 28 May 1985. [Google Scholar]
- Ezzoddin, S.; Abbasian, A.; Aman-Alikhani, M.; Ganjali, S.T. The influence of noncarcinogenic petroleum-based process oils on tire compounds’ performance. Iran. Polym. J. 2013, 22, 697–707. [Google Scholar] [CrossRef]
- Sökmen, S.; Oßwald, K.; Reincke, K.; Ilisch, S. Influence of treated distillate aromatic extract (TDAE) content and addition time on rubber-filler interactions in silica filled SBR/BR blends. Polymers 2021, 13, 698. [Google Scholar] [CrossRef] [PubMed]
- Hwang, K.; Kim, W.; Ahn, B.; Mun, H.; Yu, E.; Kim, D.; Ryu, G.; Kim, W. Effect of surfactant on the physical properties and crosslink density of silica filled ESBR compounds and carbon black filled compounds. Elast. Comp. 2018, 53, 39–47. [Google Scholar]
- Corman, B.G.; Deviney, M.L., Jr.; Whittington, L.E. The migration of extender oil in natural and synthetic rubber. IV. Effect of saturates geometry and carbon black type on diffusion rates. Rubber Chem. Technol. 1970, 43, 1349–1358. [Google Scholar] [CrossRef]
- Kim, D.; Ahn, B.; Kim, K.; Lee, J.; Kim, I.J.; Kim, W. Effects of molecular weight of functionalized liquid butadiene rubber as a processing aid on the properties of SSBR/silica compounds. Polymers 2021, 13, 850. [Google Scholar] [CrossRef]
- Ikeda, K. Bio liquid polymer for winter tires. In Proceedings of the Tire Technology EXPO 2018, Hannover, Germany, 14–16 February 2017; pp. 105–127. [Google Scholar]
- Iz, M.; Kim, D.; Hwang, K.; Kim, W.; Ryu, G.; Song, S.; Kim, W. The effects of liquid butadiene rubber and resins as processing aids on the physical properties of SSBR/silica compounds. Elast. Comp. 2020, 55, 289–299. [Google Scholar]
- Gruendken, M. Liquid rubber for safer and faster tires. In Proceedings of the Tire Technology EXPO 2018, Hannover, Germany, 14–16 February 2017. [Google Scholar]
- Sierra, V.P.; Wagemann, J.; Van De Pol, C.; Kendziorra, N.; Herzog, K.; Recker, C.; Mueller, N. Rubber Blend with Improved Rolling Resistance Behavior. U.S. Patent 9,080,042, 14 July 2015. [Google Scholar]
- Kitamura, T.; Lawson, D.F.; Morita, K.; Ozawa, Y. Anionic Polymerization Initiators and Reduced Hysteresis Products Therefrom. U.S. Patent 5,393,721, 28 February 1995. [Google Scholar]
- Cray Valley, Technical Data Sheet, Recon. Available online: http://www.crayvalley.com/docs/tds/ricon-603-.pdf?sfvrsn=2 (accessed on 9 April 2018).
- Evonik. Less Fuel and Lower CO2 Emissions with POLYVEST ST Tires. Available online: https://coatings.evonik.com/en/less-fuel-andlower-CO2-emissions-with-polyvest-st-tires100233.html (accessed on 13 February 2017).
- Herpich, R.; Fruh, T.; Heiliger, L.; Schilling, K. Silica Gel-Containing Rubber Compounds with Organosilicon Compounds as Compounding Agent. U.S. Patent 6,593,418, 15 July 2003. [Google Scholar]
- Takuya, H.; Tochiro, M. Tire Tread Rubber Composition. J.P. Patent 2,005,146,115, 14 November 2003. [Google Scholar]
- Satoyuki, S.; Chikashi, Y. Rubber Composition Containing Compound Having Organosilicon Function Group through Urethane Bond at Terminal. J.P. Patent 2,005,350,603, 22 December 2005. [Google Scholar]
- Kim, D.; Yeom, G.; Joo, H.; Ahn, B.; Paik, H.J.; Jeon, H.; Kim, W. Effect of the functional group position in functionalized liquid butadiene rubbers used as processing aids on the properties of silica-filled rubber compounds. Polymers 2021, 13, 2698. [Google Scholar] [CrossRef]
- Tezuka, Y. Telechelic polymers. Prog. Polymer Sci. 1992, 17, 471–514. [Google Scholar] [CrossRef]
- Lai, J.T.; Filla, D.; Shea, R. Functional polymers from novel carboxyl-terminated trithiocarbonates as highly efficient RAFT agents. Macromolecules 2002, 35, 6754–6756. [Google Scholar] [CrossRef]
- Hoffman, R.F.; Gobran, R.H. Liquid carboxyl-terminated poly(butadiene). Rubber Chem. Technol. 1973, 46, 139–147. [Google Scholar] [CrossRef]
- Berenbaum, M.B.; Bulbenko, G.F.; Gobran, R.H.; Hoffman, R.F. Liquid Carboxy-Terminated Polymers and Preparation Thereof with Dicarboxylic Acid Peroxides. U.S. Patent 3,235,589, 15 February 1966. [Google Scholar]
- Buback, M.; Huckestein, B.; Kuchta, F.D.; Russell, G.T.; Schmid, E. Initiator efficiencies in 2, 2′-azoisobutyronitrile-initiated free-radical polymerizations of styrene. Macromol. Chem. Phys. 1994, 195, 2117–2140. [Google Scholar] [CrossRef]
- Choi, Y.T.; El-Aasser, M.S.; Sudol, E.D.; Vanderhoff, J.W. Polymerization of styrene miniemulsions. J. Polymer Sci. Polymer Chem. Ed. 1985, 23, 2973–2987. [Google Scholar] [CrossRef]
- Pesetskii, S.S.; Jurkowski, B.; Krivoguz, Y.M.; Kelar, K. Free-radical grafting of itaconic acid onto LDPE by reactive extrusion: I. Effect of initiator solubility. Polymer 2001, 42, 469–475. [Google Scholar] [CrossRef]
- Ramier, J.; Gauthier, C.; Chazeau, L.; Stelandre, L.; Guy, L. Payne effect in silica-filled styrene-butadiene rubber: Influence of surface treatment. J. Polymer Sci. B 2007, 45, 286–298. [Google Scholar] [CrossRef]
- Lee, J.Y.; Park, N.; Lim, S.; Ahn, B.; Kim, W.; Moon, H.; Paik, H.J.; Kim, W. Influence of the silanes on the crosslink density and crosslink structure of silica-filled solution styrene butadiene rubber compounds. Comp. Interfaces 2017, 24, 711–727. [Google Scholar] [CrossRef]
- Boonstra, B.B.; Taylor, G.L. Swelling of filled rubber vulcanizates. Rubber Chem. Technol. 1965, 38, 943–960. [Google Scholar] [CrossRef]
- Verbruggen, M.A.L.; Van Der Does, L.; Noordermeer, J.W.; Van Duin, M.; Manuel, H.J. Mechanisms involved in the recycling of NR and EPDM. Rubber Chem. Technol. 1999, 72, 731–740. [Google Scholar] [CrossRef]
- Derouet, D.; Forgeard, S.; Brosee, J. Synthesis of alkoxysilyl-terminated polyisoprenes by means of ‘living’anionic polymerization, 2. Synthesis of trialkoxysilyl-terminated 1, 4polyisoprenes by reaction of polyisoprenyllithium with various functional trialkoxysilanes selected as end-capping reagents. Macromol. Chem. Phys. 1999, 200, 10–24. [Google Scholar]
- Liu, X.; Zhao, S.; Zhang, X.; Li, X.; Bai, Y. Preparation, structure, and properties of solutionpolymerized styrene-butadiene rubber with functionalized end-groups and its silica-filled composites. Polymer 2014, 55, 1964–1976. [Google Scholar] [CrossRef]
- Jayalakshmy, M.S.; Mishra, R.K. Applications of carbon-based nanofillerincorporated rubber composites in the fields of tire engineering, flexible electronics and EMI shielding. In Carbon-Based Nanofillers and Their Rubber Nanocomposites; Elsevier: Amsterdam, The Netherlands, 2019; pp. 441–472. [Google Scholar]
- de Monredon-Senani, S.; Bonhomme, C.; Ribot, F.; Babonneau, F. Covalent grafting of organoalkoxysilanes on silica surfaces in water-rich medium as evidenced by 29Si NMR. J. Sol-Gel Sci. Technol. 2009, 50, 152–157. [Google Scholar] [CrossRef]
- Sugiyama, T.; Shiba, H.; Yoshikawa, M.; Wada, H.; Shimojima, A.; Kuroda, K. Synthesis of polycyclic and cage siloxanes by hydrolysis and intramolecular condensation of alkoxysilylated cyclosiloxanes. Chem. Eur. J. 2019, 25, 2764–2772. [Google Scholar] [CrossRef]
- Choi, S.S.; Kim, I.S.; Woo, C.S. Influence of TESPT content on crosslink types and rheological behaviors of natural rubber compounds reinforced with silica. J. Appl. Polymer Sci. 2007, 106, 2753–2758. [Google Scholar] [CrossRef]
- Hwang, K.; Song, S.; Kang, Y.Y.; Suh, J.; Jeon, H.B.; Kwag, G.; Paik, H.J.; Kim, W. Effect of emulsion SBR prepared by asymmetric reversible addition fragmentation transfer agent on properties of silica-filled compounds. Rubber Chem. Technol. 2021, 94, 735–758. [Google Scholar] [CrossRef]
- Zhao, F.; Bi, W.; Zhao, S. Influence of crosslink density on mechanical properties of natural rubber vulcanizates. J. Macromol. Sci. B 2011, 50, 1460–1469. [Google Scholar] [CrossRef]
- Ryu, G.; Kim, D.; Song, S.; Hwang, K.; Kim, W. Effect of the epoxide contents of liquid isoprene rubber as a processing aid on the properties of silica-filled natural rubber compounds. Polymers 2021, 13, 3026. [Google Scholar] [CrossRef] [PubMed]
- Halasa, A.F.; Prentis, J.; Hsu, B.; Jasiunas, C. High vinyl high styrene solution SBR. Polymer 2005, 46, 4166–4174. [Google Scholar] [CrossRef]
- Isitman, N.A.H.; Thielen, G.M.V. Rubber Composition and Pneumatic Tire. E.U. Patent 3450490A1, 6 August 2018. [Google Scholar]
- Hirata, K.; Moriguchi, M. Bio-based liquid rubber for tire application. Rubber World 2017, 256, 50–55. [Google Scholar]
- Derham, C.F.; Newell, R.; Swift, P.M. The use of silica for improving tread grip in winter tires. NR Technol. 1988, 19, 1–9. [Google Scholar]
- Dörrie, H.; Schröder, C.; Wies, B. Winter tires: Operating conditions, tire characteristics and vehicle driving behavior. Tire Sci. Technol. 2010, 38, 119–136. [Google Scholar] [CrossRef]
- Wang, M.J. Effect of polymer-filler and filler-filler interactions on dynamic properties of filled vulcanizates. Rubber Chem. Technol. 1998, 71, 520–589. [Google Scholar] [CrossRef]
- Warasitthinon, N.; Robertson, C.G. Interpretation of the tan δ peak height for particle-filled rubber and polymer nanocomposites with relevance to tire tread performance balance. Rubber Chem. Technol. 2018, 91, 577–594. [Google Scholar] [CrossRef]
- Wang, M.J.; Kutsovsky, Y.; Zhang, P.; Murphy, L.J.; Laube, S.; Mahmud, K. New generation carbon-silica dual phase filler part I. Characterization and application to passenger tire. Rubber Chem. Technol. 2002, 75, 247–263. [Google Scholar] [CrossRef]
- Wang, M.J.; Zhang, P.; Mahmud, K. Carbon-silica dual phase filler, a new generation reinforcing agent for rubber: Part IX. Application to truck tire tread compound. Rubber Chem. Technol. 2001, 74, 124–137. [Google Scholar] [CrossRef]












| Organic Compounds | 2-Azo-LqBR | 4-Azo-LqBR | |
|---|---|---|---|
| Di-functional initiator | 2-Azo-initiator | 2.183 | - |
| Tetra-functional initiator | 4-Azo-initiator | - | 1.217 |
| Monomer | 1,3-butadiene | 60 | 60 |
| Solvent | THF | 420 | 300 |
| Sample | TDAE Oil | N-LqBR | 2-Azo-LqBR | 4-Azo-LqBR |
|---|---|---|---|---|
| SSBR | 80 | 80 | 80 | 80 |
| BR | 20 | 20 | 20 | 20 |
| Silica | 120 | 120 | 120 | 120 |
| X50S | 20 | 20 | 20 | 20 |
| DPG | 2 | 2 | 2 | 2 |
| TDAE oil | 40 | 30 | 30 | 30 |
| N-LqBR | - | 10 | - | - |
| 2-Azo-LqBR | - | - | 10 | - |
| 4-Azo-LqBR | - | - | - | 10 |
| Wax | 1 | 1 | 1 | 1 |
| TMQ | 1 | 1 | 1 | 1 |
| ZnO | 3 | 3 | 3 | 3 |
| Stearic acid | 1 | 1 | 1 | 1 |
| 6PPD | 2 | 2 | 2 | 2 |
| Sulfur | 1.3 | 1.3 | 1.3 | 1.3 |
| CBS | 1.6 | 1.6 | 1.6 | 1.6 |
| ZBEC | 0.1 | 0.1 | 0.1 | 0.1 |
| Time, mins | RPM | Action | |
|---|---|---|---|
| Silica masterbatch (SMB) mixing | 00:00–00:40 | 20 | Add rubber (initial temp.: 110 °C) |
| 00:40–01:40 | 40 | Add silica 1/2, X50S 1/2, DPG 1/2, processing aid 1/2 | |
| 01:40–02:40 | 40 | Add silica 1/2, X50S 1/2, DPG 1/2, processing aid 1/2 | |
| 02:40–05:00 | 60 | Add additives | |
| 05:00–05:30 | 60 | Ram up | |
| 05:30–07:40 | 50 | Extra mixing and dump (dump temp.: 150–155 °C) | |
| Final masterbatch (FMB) mixing | 00:00–00:20 | 20 | Add SMB (initial temp.: 50 °C) |
| 00:20–02:00 | 40 | Add sulfur, CBS, ZBEC and dump (dump temp.: 80–90 °C) |
| Property | Unit | N-LqBR | 2-Azo-LqBR | 4-Azo-LqBR |
|---|---|---|---|---|
| Sample Mn | g/mol | 4400 | 4000 | 5100 |
| Polydispersity index (Đ) | - | 1.04 | 1.20 | 1.32 |
| Vinyl content (% in BD) | - | 15 | 19 | 19 |
| Tg | °C | −95 | −86 | −78 |
| End functionality (Si per chain) | - | 0 | 2.3 | 4.3 |
| Property | TDAE Oil | N-LqBR | 2-Azo-LqBR | 4-Azo-LqBR |
|---|---|---|---|---|
| G′ (at 0.28% strain) | 8.98 | 8.37 | 3.69 | 4.96 |
| G′ (at 40.04% strain) | 0.36 | 0.36 | 0.40 | 0.41 |
| ΔG′ (at 0.28–40.04% strain) | 8.62 | 8.01 | 3.29 | 4.55 |
| Property | Unit | TDAE Oil | N-LqBR | 2-Azo-LqBR | 4-Azo-LqBR |
|---|---|---|---|---|---|
| Mooney viscosity (ML1+4 at 100 °C, FMB) | MU | 153 | 147 | 116 | 119 |
| Tmin | N-m | 0.88 | 0.82 | 0.68 | 0.77 |
| Tmax | N-m | 3.02 | 2.65 | 2.88 | 2.85 |
| Δtorque | N-m | 2.14 | 1.83 | 2.20 | 2.09 |
| t10 | min:sec | 1:11 | 1:05 | 1:09 | 1:15 |
| t90 | min:sec | 10:02 | 11:55 | 10:40 | 11:51 |
| Property | Unit | TDAE Oil | N-LqBR | 2-Azo-LqBR | 4-Azo-LqBR |
|---|---|---|---|---|---|
| Weight loss after extraction | % | 15.66 | 14.96 | 12.33 | 12.42 |
| Weight loss after extraction in 10 phr of oil and LqBR | % | 100 | 79.5 | 2.2 | 4.8 |
| Crosslink density | 10−4 mol/g | 1.42 | 1.27 | 1.75 | 1.65 |
| Property | Unit | TDAE Oil | N-LqBR | 2-Azo-LqBR | 4-Azo-LqBR |
|---|---|---|---|---|---|
| M100 | kgf/cm2 | 46 | 43 | 55 | 50 |
| M300 | kgf/cm2 | 142 | 135 | 168 | 163 |
| Elongation at break | % | 335 | 334 | 315 | 305 |
| Tensile strength | kgf/cm2 | 161 | 151 | 178 | 167 |
| DIN abrasion loss | mg | 109 | 96 | 91 | 93 |
| Property | Unit | TDAE Oil | N-LqBR | 2-Azo- LqBR | 4-Azo- LqBR |
|---|---|---|---|---|---|
| Number end functional groups | - | N/A | 0 | 2 | 4 |
| Tg | °C | −43.1 | −45.9 | −44.6 | −44.8 |
| E′ at −30 °C | MPa | 164 | 163 | 137 | 152 |
| Tan δ at 60 °C (0.2% strain, temperature sweep) | - | 0.177 | 0.188 | 0.165 | 0.170 |
| Tan δ at 60 °C (5% strain, strain sweep) | - | 0.196 | 0.207 | 0.176 | 0.190 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, S.; Choi, H.; Jeong, J.; Kim, S.; Kwon, M.; Kim, M.; Kim, D.; Jeon, H.; Paik, H.-j.; Chung, S.; et al. Optimized End Functionality of Silane-Terminated Liquid Butadiene Rubber for Silica-Filled Rubber Compounds. Polymers 2023, 15, 2583. https://doi.org/10.3390/polym15122583
Song S, Choi H, Jeong J, Kim S, Kwon M, Kim M, Kim D, Jeon H, Paik H-j, Chung S, et al. Optimized End Functionality of Silane-Terminated Liquid Butadiene Rubber for Silica-Filled Rubber Compounds. Polymers. 2023; 15(12):2583. https://doi.org/10.3390/polym15122583
Chicago/Turabian StyleSong, Sanghoon, Haeun Choi, Junhwan Jeong, Seongyoon Kim, Myeonghee Kwon, Minji Kim, Donghyuk Kim, Heungbae Jeon, Hyun-jong Paik, Sungwook Chung, and et al. 2023. "Optimized End Functionality of Silane-Terminated Liquid Butadiene Rubber for Silica-Filled Rubber Compounds" Polymers 15, no. 12: 2583. https://doi.org/10.3390/polym15122583