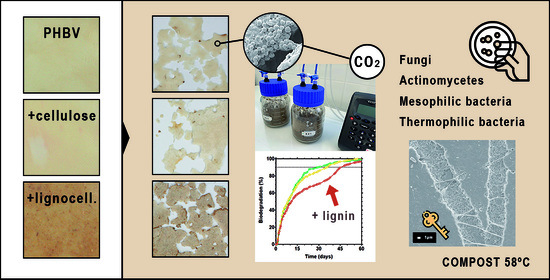
Effect of the Presence of Lignin from Woodflour on the Compostability of PHA-Based Biocomposites: Disintegration, Biodegradation and Microbial Dynamics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Fiber/PHBV Composites
2.3. Characterization of Fibers and Composites
2.4. Biodegradability Assessment
3. Results and Discussion
3.1. Characterization of Fibers and Composites
3.2. Biodegradation Assessment
3.3. Microbial Dynamics over the Composting Process
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dietrich, K.; Dumont, M.J.; Orsat, V.; Del Rio, L.F. In-depth material characterization of polyhydroxybutyrate from a forest biorefinery. J. Appl. Polym. Sci. 2021, 138, 51375. [Google Scholar] [CrossRef]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef]
- Fan, Y.V.; Jiang, P.; Tan, R.R.; Aviso, K.B.; You, F.; Zhao, X.; Lee, C.T.; Klemes, J.J. Forecasting plastic waste generation and interventions for environmental hazard mitigation. J. Hazard. Mater. 2022, 424, 127330. [Google Scholar] [CrossRef] [PubMed]
- Degli-Innocenti, F.; Breton, T. Intrinsic biodegradability of plastics and ecological risk in the case of leakage. ACS Sustain. Chem. Eng. 2020, 8, 9239–9249. [Google Scholar] [CrossRef]
- Sánchez-Safont, E.L.; Aldureid, A.; Lagarón, J.M.; Gámez-Pérez, J.; Cabedo, L. Biocomposites of different lignocellulosic wastes for sustainable food packaging applications. Compos. B Eng. 2018, 145, 215–225. [Google Scholar] [CrossRef]
- Rivera-Briso, A.L.; Serrano-Aroca, A. poly(3-hydroxybutyrate-co-3-hydroxyvalerate): Enhancement strategies for advanced applications. Polymers 2018, 10, 732. [Google Scholar] [CrossRef]
- Adeleye, A.T.; Odoh, C.K.; Enudi, O.C.; Banjoko, O.O.; Osiboye, O.O.; Toluwalope Odediran, E.; Louis, H. Sustainable synthesis and applications of polyhydroxyalkanoates (PHAs) from biomass. Process Biochem. 2020, 96, 174–193. [Google Scholar] [CrossRef]
- De Donno Novelli, L.; Moreno Sayavedra, S.; Rene, E.R. Polyhydroxyalkanoate (PHA) production via resource recovery from industrial waste streams: A review of techniques and perspectives. Bioresour. Technol. 2021, 331, 124985. [Google Scholar] [CrossRef]
- Emadian, S.M.; Onay, T.T.; Demirel, B. Biodegradation of bioplastics in natural environments. Waste Manag. 2017, 59, 526–536. [Google Scholar] [CrossRef]
- Bátori, V.; Åkesson, D.; Zamani, A.; Taherzadeh, M.J.; Sárvári Horváth, I. Anaerobic degradation of bioplastics: A review. Waste Manag. 2018, 80, 406–413. [Google Scholar] [CrossRef]
- Rudnik, E. Biodegradation of compostable polymers in various environments. In Compostable Polymer Materials, 2nd ed.; Elsevier: Boston, MA, USA, 2019; Chapter 8; pp. 255–292. [Google Scholar] [CrossRef]
- Crutchik, D.; Franchi, O.; Caminos, L.; Jeison, D.; Belmonte, M.; Pedrouso, A.; Val del Río, A.; Mosquera-Corral, A.; Campos, J.L. Polyhydroxyalkanoates (PHAs) production: A feasible economic option for the treatment of sewage sludge in municipal wastewater treatment plants? Water 2020, 12, 1118. [Google Scholar] [CrossRef]
- Kumar, V.; Sehgal, R.; Gupta, R. Blends and composites of polyhydroxyalkanoates (PHAs) and their applications. Eur. Polym. J. 2021, 161, 110824. [Google Scholar] [CrossRef]
- Srubar, W.V.; Pilla, S.; Wright, Z.C.; Ryan, C.A.; Greene, J.P.; Frank, C.W.; Billington, S.L. Mechanisms and impact of fiber–matrix compatibilization techniques on the material characterization of PHBV/oak wood flour engineered biobased composites. Compos. Sci. Technol. 2012, 72, 708–715. [Google Scholar] [CrossRef]
- Brandt, A.; Gräsvik, J.; Hallett, J.P.; Welton, T. Deconstruction of lignocellulosic biomass with ionic liquids. Green Chem. 2013, 15, 550–583. [Google Scholar] [CrossRef]
- Barker, B.; Owen, N.L. Identifying softwoods and hardwoods by infrared spectroscopy. J. Chem. Edu. 1999, 76, 1706. [Google Scholar] [CrossRef]
- Grillo Fernandes, E.; Pietrini, M.; Chiellini, E. Bio-based polymeric composites comprising wood flour as filler. Biomacromolecules 2004, 5, 1200–1205. [Google Scholar] [CrossRef]
- Shibata, M.; Takachiyo, K.I.; Ozawa, K.; Yosomiya, R.; Takeishi, H. Biodegradable polyester composites reinforced with short abaca fiber. J. Appl. Polym. Sci. 2002, 85, 129–138. [Google Scholar] [CrossRef]
- Wu, C.S.; Liao, H.T. Fabrication, characterization, and application of polyester/wood flour composites. J. Polym. Eng. 2017, 37, 689–698. [Google Scholar] [CrossRef]
- Singh, S.; Mohanty, A.K.; Sugie, T.; Takai, Y.; Hamada, H. Renewable resource based biocomposites from natural fiber and polyhydroxybutyrate-co-valerate (PHBV) bioplastic. Compos. -A Appl. Sci. Manuf. 2008, 39, 875–886. [Google Scholar] [CrossRef]
- Thomas, S.; Shumilova, A.; Kiselev, E.; Baranovsky, S.; Vasiliev, A.; Nemtsev, I.; Kuzmin, A.P.; Sukovatyi, A.; Avinash, R.P.; Volova, T. Thermal, mechanical and biodegradation studies of biofiller based poly-3-hydroxybutyrate biocomposites. Int. J. Biol. Macromol. 2020, 155, 1373–1384. [Google Scholar] [CrossRef]
- Wu, C.S.; Liao, H.T.; Cai, Y.X. Characterisation, biodegradability and application of palm fibre-reinforced polyhydroxyalkanoate composites. Polym. Degrad. Stab. 2017, 140, 55–63. [Google Scholar] [CrossRef]
- Avella, M.; La Rota, G.; Martuscelli, E.; Raimo, M.; Sadocco, P.; Elegir, G.; Riva, R. Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) and wheat straw fibre composites: Thermal, mechanical properties and biodegradation behaviour. J. Mater. Sci. 2000, 35, 829–836. [Google Scholar] [CrossRef]
- Chan, C.M.; Vandi, L.J.; Pratt, S.; Halley, P.; Richardson, D.; Werker, A.; Laycock, B. Insights into the biodegradation of PHA/wood composites: Micro- and macroscopic changes. Sustain. Mater. Technol. 2019, 21, e00099. [Google Scholar] [CrossRef]
- Muniyasamy, S.; Ofosu, O.; Thulasinathan, B.; Thondi Rajan, A.S.; Ramu, S.M.; Soorangkattan, S.; Muthuramalingam, J.B.; Alagarsamy, A. Thermal- chemical and biodegradation behaviour of alginic acid treated flax fibers/poly (hydroxybutyrate-co-valerate) PHBV green composites in compost medium. Biocatal Agric Biotechnol 2019, 22, 101394. [Google Scholar] [CrossRef]
- Vannini, C.; Rossi, A.; Vallerini, F.; Menicagli, V.; Seggiani, M.; Cinelli, P.; Lardicci, C.; Balestri, E. Microbial communities of polyhydroxyalkanoate (PHA)-based biodegradable composites plastisphere and of surrounding environmental matrix: A comparison between marine (seabed) and coastal sediments (dune sand) over a long-time scale. Sci. Total Environ. 2021, 764, 142814. [Google Scholar] [CrossRef]
- Sun, Y.; Ren, X.; Rene, E.R.; Wang, Z.; Zhou, L.; Zhang, Z.; Wang, Q. The degradation performance of different microplastics and their effect on microbial community during composting process. Bioresour. Technol. 2021, 332, 125133. [Google Scholar] [CrossRef] [PubMed]
- García-Depraect, O.; Bordel, S.; Lebrero, R.; Santos-Beneit, F.; Börner, R.A.; Börner, T.; Muñoz, R. Inspired by nature: Microbial production, degradation and valorization of biodegradable bioplastics for life-cycle-engineered products. Biotechnol. Adv. 2021, 53, 107772. [Google Scholar] [CrossRef]
- Lim, B.K.H.; Thian, E.S. Biodegradation of polymers in managing plastic waste—A review. Sci. Total Environ. 2022, 813, 151880. [Google Scholar] [CrossRef]
- Gadaleta, G.; De Gisi, S.; Picuno, C.; Heerenklage, J.; Cafiero, L.; Oliviero, M.; Notarnicola, M.; Kuchta, K.; Sorrentino, A. The influence of bio-plastics for food packaging on combined anaerobic digestion and composting treatment of organic municipal waste. Waste Manag. 2022, 144, 87–97. [Google Scholar] [CrossRef]
- Lammi, S.; Gastaldi, E.; Gaubiac, F.; Angellier-Coussy, H. How olive pomace can be valorized as fillers to tune the biodegradation of PHBV-based composites. Polym. Degrad. Stab. 2019, 166, 325–333. [Google Scholar] [CrossRef]
- Dilawar, H.; Eskicioglu, C. Laboratory and field scale biodegradability assessment of biocomposite cellphone cases for end-of-life management. Waste Manag. 2022, 138, 148–157. [Google Scholar] [CrossRef]
- Narancic, T.; Verstichel, S.; Reddy Chaganti, S.; Morales-Gamez, L.; Kenny, S.T.; De Wilde, B.; Babu Padamati, R.; O’Connor, K.E. Biodegradable plastic blends create new possibilities for end-of-life management of plastics but they are not a panacea for plastic pollution. Environ. Sci. Technol. 2018, 52, 10441–10452. [Google Scholar] [CrossRef]
- ISO 20200:2015; Plastics—Determination of the Degree of Disintegration of Plastic Materials under Simulated Composting Conditions in a Laboratory-Scale Test. International Organization for Standardization:: Geneva, Switzerland, 2015.
- Bassett, D. Developments in Crystalline Polymers—2; Springer Netherlands: Dordrecht, Netherlands, 2012. [Google Scholar]
- Pamies, R.; Hernández Cifre, J.G.; López Martínez, M.C.; García de la Torre, J. Determination of intrinsic viscosities of macromolecules and nanoparticles. Comparison of single-point and dilution procedures. Colloid Polym. Sci. 2008, 286, 1223–1231. [Google Scholar] [CrossRef]
- López Martínez, M.C.; Díaz Baños, F.G.; Ortega Retuerta, A.; García de la Torre, J. Multiple linear least-squares fits with a common intercept: Determination of the intrinsic viscosity of macromolecules in solution. J. Chem. Edu. 2003, 80, 1036. [Google Scholar] [CrossRef]
- ISO 14855-1:2012; Determination of the Ultimate Aerobic Biodegradability of Plastic Materials under Controlled Composting Conditions—Method by Analysis of Evolved Carbon Dioxide—Part 1: General Method. International Organization for Standardization: Geneva, Switzerland, 2012.
- Herrera, R.; Erdocia, X.; Llano-Ponte, R.; Labidi, J. Characterization of hydrothermally treated wood in relation to changes on its chemical composition and physical properties. J. Anal. Appl. Pyrolysis 2014, 107, 256–266. [Google Scholar] [CrossRef]
- French, A.D. Idealized powder diffraction patterns for cellulose polymorphs. Cellulose. 2014, 21, 885–896. [Google Scholar] [CrossRef]
- Miao, X.; Lin, J.; Bian, F. Utilization of discarded crop straw to produce cellulose nanofibrils and their assemblies. J. Bioresour. Bioprod. 2020, 5, 26–36. [Google Scholar] [CrossRef]
- Tranvan, L.; Legrand, V.; Jacquemin, F. Thermal decomposition kinetics of balsa wood: Kinetics and degradation mechanisms comparison between dry and moisturized materials. Polym. Degrad. Stab. 2014, 110, 208–215. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, S.; Wang, Q.; Ji, X.; Yang, G.; Chen, J.; Fatehi, P. Strong, ductile and biodegradable polylactic acid/lignin-containing cellulose nanofibril composites with improved thermal and barrier properties. Ind. Crops Prod. 2021, 171, 113898. [Google Scholar] [CrossRef]
- Vanska, E.; Vihela, T.; Peresin, M.S.; Vartiainen, J.; Hummel, M.; Vuorinen, T. Residual lignin inhibits thermal degradation of cellulosic fiber sheets. Cellulose. 2016, 23, 199–212. [Google Scholar] [CrossRef]
- Abidi, N.; Cabrales, L.; Haigler, C.H. Changes in the cell wall and cellulose content of developing cotton fibers investigated by FTIR spectroscopy. Carbohydr. Polym. 2014, 100, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Li, Q.; Shi, Z.; Ye, J. Interactions between wheat starch and cellulose derivatives in short-term retrogradation: Rheology and FTIR study. Food Res. Int. 2017, 100, 858–863. [Google Scholar] [CrossRef] [PubMed]
- Colom, X.; Carrillo, F. Comparative study of wood samples of the northern area of Catalonia by FTIR. J. Wood Chem. Technol. 2005, 25, 1–11. [Google Scholar] [CrossRef]
- Gelbrich, J.; Mai, C.; Militz, H. Evaluation of bacterial wood degradation by Fourier Transform Infrared (FTIR) measurements. J. Cult. Herit. 2012, 13, S135–S138. [Google Scholar] [CrossRef]
- Sánchez-Safont, E.L.; González-Ausejo, J.; Gámez-Pérez, J.; Lagarón, J.M.; Cabedo, L. Poly(3-hydroxybutyrate-co-3-hydroxyvalerate)/purified cellulose fiber composites by melt blending: Characterization and degradation in composting conditions. J. Renew. Mater. 2016, 4, 123–132. [Google Scholar] [CrossRef]
- Kunioka, M.; Doi, Y. Thermal degradation of microbial copolyesters: Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) and poly(3-hydroxybutyrate-co-4-hydroxybutyrate). Macromolecules 1990, 23, 1933–1936. [Google Scholar] [CrossRef]
- Abe, H. Thermal degradation of environmentally degradable poly(hydroxyalkanoic acid)s. Macromol. Biosci. 2006, 6, 469–486. [Google Scholar] [CrossRef]
- Chen, C.; Fei, B.; Peng, S.; Zhuang, Y.; Dong, L.; Feng, Z. The kinetics of the thermal decomposition of poly(3-hydroxybutyrate) and maleated poly(3-hydroxybutyrate). J. Appl. Polym. Sci. 2002, 84, 1789–1796. [Google Scholar] [CrossRef]
- Bledzki, A.; Jaszkiewicz, A. Mechanical performance of biocomposites based on PLA and PHBV reinforced with natural fibres—A comparative study to PP. Compos. Sci. Technol. 2010, 70, 1687–1696. [Google Scholar] [CrossRef]
- Fraga, A.; Ruseckaite, R.A.; Jiménez, A. Thermal degradation and pyrolysis of mixtures based on poly(3-hydroxybutyrate-8%-3-hydroxyvalerate) and cellulose derivatives. Polym. Test. 2005, 24, 526–534. [Google Scholar] [CrossRef]
- Pal, A.K.; Wu, F.; Misra, M.; Mohanty, A.K. Reactive extrusion of sustainable PHBV/PBAT-based nanocomposite films with organically modified nanoclay for packaging applications: Compression moulding vs. cast film extrusion. Compos. B Eng. 2020, 198, 108141. [Google Scholar] [CrossRef]
- Gupta, A.; Simmons, W.; Schueneman, G.T.; Hylton, D.; Mintz, E.A. Rheological and thermo-mechanical properties of poly(lactic acid)/lignin-coated cellulose nanocrystal composites. ACS Sustain. Chem. Eng. 2017, 5, 1711–1720. [Google Scholar] [CrossRef]
- Luo, S.; Cao, J.; McDonald, A.G. Esterification of industrial lignin and its effect on the resulting poly(3-hydroxybutyrate-co-3-hydroxyvalerate) or polypropylene blends. Ind. Crops Prod. 2017, 97, 281–291. [Google Scholar] [CrossRef]
- Larrañaga, A.; Lizundia, E. A review on the thermomechanical properties and biodegradation behaviour of polyesters. Eur. Polym. J. 2019, 121, 109296. [Google Scholar] [CrossRef]
- Puglia, D.; Fortunati, E.; D’Amico, D.; Manfredi, L.; Cyras, V.; Kenny, J. Influence of organically modified clays on the properties and disintegrability in compost of solution cast poly(3-hydroxybutyrate) films. Polym. Degrad. Stab. 2014, 99, 127–135. [Google Scholar] [CrossRef]
- Rudnik, E. Biodegradability testing of compostable polymer materials under laboratory conditions. In Compostable Polymer Materials, 2nd ed.; Elsevier: Boston, MA, USA, 2019; Chapter 6; pp. 163–237. [Google Scholar] [CrossRef]
- Mueller, R.J. Biological degradation of synthetic polyesters—Enzymes as potential catalysts for polyester recycling. Process biochem. 2006, 41, 2124–2128. [Google Scholar] [CrossRef]
- Kijchavengkul, T.; Auras, R. Compostability of polymers. Polym. Int. 2008, 57, 793–804. [Google Scholar] [CrossRef]
- Tokiwa, Y.; Calabia, B.P. Biodegradability and biodegradation of polyesters. J. Polym. Environ. 2007, 15, 259–267. [Google Scholar] [CrossRef]
- Polman, E.M.; Gruter, G.J.M.; Parsons, J.R.; Tietema, A. Comparison of the aerobic biodegradation of biopolymers and the corresponding bioplastics: A review. Sci. Total Environ. 2021, 753, 141953. [Google Scholar] [CrossRef]
- Ibrahim, H.; Mehanny, S.; Darwish, L.; Farag, M. A comparative study on the mechanical and biodegradation characteristics of starch-based composites reinforced with different lignocellulosic fibers. J. Polym. Environ. 2018, 26, 2434–2447. [Google Scholar] [CrossRef]
- Lv, S.; Zhang, Y.; Gu, J.; Tan, H. Biodegradation behavior and modelling of soil burial effect on degradation rate of PLA blended with starch and wood flour. Colloids Surf. B 2017, 159, 800–808. [Google Scholar] [CrossRef]
- Asina, F.; Brzonova, I.; Voeller, K.; Kozliak, E.; Kubátová, A.; Yao, B.; Ji, Y. Biodegradation of lignin by fungi, bacteria and laccases. Bioresour. Technol. 2016, 220, 414–424. [Google Scholar] [CrossRef]
- ISO 17088:2008; Specifications for Compostable Plastics. International Organization for Standardization: Geneva, Switzerland, 2021.
- EN 13432:2000; Packaging—Requirements for Packaging Recoverable through Composting and Biodegradation—Test Scheme and Evaluation Criteria for the Final Acceptance of Packaging. International Organization for Standardization: Geneva, Switzerland, 2000.
- Maity, S.; Banerjee, S.; Biswas, C.; Guchhait, R.; Chatterjee, A.; Pramanick, K. Functional interplay between plastic polymers and microbes: A comprehensive review. Biodegradation 2021, 32, 487–510. [Google Scholar] [CrossRef]
- Galitskaya, P.; Biktasheva, L.; Saveliev, A.; Grigoryeva, T.; Boulygina, E.; Selivanovskaya, S. Fungal and bacterial successions in the process of co-composting of organic wastes as revealed by 454 pyrosequencing. PLoS ONE 2017, 12, e0186051. [Google Scholar] [CrossRef] [PubMed]
- Wright, C.; Gryganskyi, A.P.; Bonito, G. Fungi in composting. In Fungal Applications in Sustainable Environmental Biotechnology, 1st ed.; Purchase, D., Ed.; Springer: Cham, Switzerland, 2016; Chapter 1; pp. 3–28. [Google Scholar] [CrossRef]
- Husárová, L.; Pekarǒvá, S.; Stloukal, P.; Kucharzcyk, P.; Verney, V.; Commereuc, S.; Ramone, A.; Koutny, M. Identification of important abiotic and biotic factors in the biodegradation of poly(l-lactic acid). Int. J. Biol. Macromol. 2014, 71, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Karamanlioglu, M.; Houlden, A.; Robson, G.D. Isolation and characterisation of fungal communities associated with degradation and growth on the surface of poly(lactic) acid (PLA) in soil and compost. Int. Biodeterior. Biodegrad. 2014, 95, 301–310. [Google Scholar] [CrossRef]
- Langarica-Fuentes, A.; Handley, P.S.; Houlden, A.; Fox, G.; Robson, G.D. An investigation of the biodiversity of thermophilic and thermotolerant fungal species in composts using culture-based and molecular techniques. Fungal Ecol. 2014, 11, 132–144. [Google Scholar] [CrossRef]
- Soleimani, Z.; Gharavi, S.; Soudi, M.; Moosavi-Nejad, Z. A survey of intact low-density polyethylene film biodegradation by terrestrial Actinobacterial species. Int. Microbiol. 2021, 24, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.A.; Hasan, F.; Hameed, A.; Ahmed, S. Isolation and characterisation of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) degrading actinomycetes and purification of PHBV depolymerase from newly isolated Streptoverticillium kashmirense AF1. Ann. Microbiol. 2007, 57, 583–588. [Google Scholar] [CrossRef]
- Butbunchu, N.; Pathom-Aree, W. Actinobacteria as promising candidate for polylactic acid type bioplastic degradation. Front. Microbiol. 2019, 10, 2834. [Google Scholar] [CrossRef]
- Volova, T.G.; Prudnikova, S.V.; Vinogradova, O.N.; Syrvacheva, D.A.; Shishatskaya, E.I. Microbial degradation of polyhydroxyalkanoates with different chemical compositions and their biodegradability. Microbial Ecol. 2017, 73, 353–367. [Google Scholar] [CrossRef] [PubMed]







| T5% (°C) | Tmax.p (°C) | Tmax.f (°C) | |
|---|---|---|---|
| Cellulose TC90 | 288 | - | 351 |
| Woodflour WF | 273 | - | 359 |
| PHBV | 277 | 297 | - |
| PHBV + TC | 276 | 294 | 344 |
| PHBV + WF | 264 | 290 | 352 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feijoo, P.; Marín, A.; Samaniego-Aguilar, K.; Sánchez-Safont, E.; Lagarón, J.M.; Gámez-Pérez, J.; Cabedo, L. Effect of the Presence of Lignin from Woodflour on the Compostability of PHA-Based Biocomposites: Disintegration, Biodegradation and Microbial Dynamics. Polymers 2023, 15, 2481. https://doi.org/10.3390/polym15112481
Feijoo P, Marín A, Samaniego-Aguilar K, Sánchez-Safont E, Lagarón JM, Gámez-Pérez J, Cabedo L. Effect of the Presence of Lignin from Woodflour on the Compostability of PHA-Based Biocomposites: Disintegration, Biodegradation and Microbial Dynamics. Polymers. 2023; 15(11):2481. https://doi.org/10.3390/polym15112481
Chicago/Turabian StyleFeijoo, Patricia, Anna Marín, Kerly Samaniego-Aguilar, Estefanía Sánchez-Safont, José M. Lagarón, José Gámez-Pérez, and Luis Cabedo. 2023. "Effect of the Presence of Lignin from Woodflour on the Compostability of PHA-Based Biocomposites: Disintegration, Biodegradation and Microbial Dynamics" Polymers 15, no. 11: 2481. https://doi.org/10.3390/polym15112481