The Synergistic Effects of Aminosilane Coupling Agent on the Adhesion Performance of Silane Primer for Silicone Resin Thermal Protection Coating
Abstract
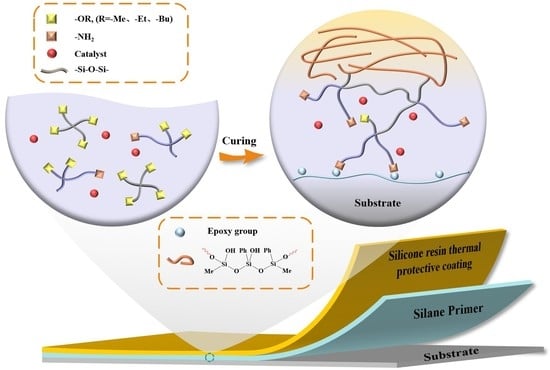
:1. Introduction
2. Experiment
2.1. Materials
2.2. Preparation and Application of Silane Primer
2.3. Characterization
3. Results and Discussion
3.1. Adhesion Properties
3.2. Surface Morphology of Silane Primer
3.3. Surface Composition of Silane Primer
3.4. Thermal Stability of Silane Primer
3.5. The Hydrolysis and Curing of the Silane Primer on the Substrate
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yao, Y.; Lu, G.Q.; Boroyevich, D.; Ngo, K.D.T. Effect of Al2O3 fibers on the high-temperature stability of silicone elastomer. Polymer 2014, 55, 4232–4240. [Google Scholar] [CrossRef]
- Fang, W.; Zeng, X.; Lai, X.; Li, H.; Chen, W.; Zhang, Y. Thermal degradation mechanism of addition-cure liquid silicone rubber with urea-containing silane. Thermochim. Acta 2015, 605, 28–36. [Google Scholar] [CrossRef]
- Raeisi, E.; Ganachaud, F.; Kang, Y.; Dong, N.; Bierwagen, G.P. Surface Analysis of Various Methods of Preparing Al 2024-T3 Surfaces for Painting. Corrosion 2000, 56, 395–400. [Google Scholar]
- Eduok, U.; Xu, Z.; Szpunar, J. Fabricating protective silica/PMDS composite films for Mg alloy: Correlating bulk silica reinforcement with barrier performance. J. Non-Cryst. Solids 2018, 485, 47–56. [Google Scholar] [CrossRef]
- Grard, A.; Belec, L.; Perrin, F.X. Effect of surface morphology on the adhesion of silicone elastomers on AA6061 aluminum alloy. Int. J. Adhes. Adhes. 2020, 102, 102656. [Google Scholar] [CrossRef]
- Tian, J.; Yan, L.; Zhang, H.; Zhou, S.; Xia, S.; Zou, H. Improving the Heat and Ablation Resistance of Silicone Rubber Composites by Incorporating Hollow Microspheres. Polymers 2022, 14, 3846. [Google Scholar] [CrossRef]
- Picard, L.; Phalip, P.; Fleury, E.; Ganachaud, F. Chemical adhesion of silicone elastomers on primed metal surfaces: A comprehensive survey of open and patent literatures. Prog. Org. Coat. 2015, 80, 120–141. [Google Scholar] [CrossRef]
- Xu, Y.; Long, J.; Zhang, R.; Du, Y.; Guan, S.; Wang, Y.; Huang, L.; Wei, H.; Liu, L.; Huang, Y. Greatly improving thermal stability of silicone resins by modification with POSS. Polym. Degrad. Stab. 2020, 174, 109082. [Google Scholar] [CrossRef]
- Su, Z.H.; Jiang, K.Z.; Zhu, X.F.; Huang, H.Y.; Yan, Y.; Pei, Y.B.; Wu, L.B. Composition Analysis of Primer for Silicone Thermal Protective Coating. Silicone Material. 2020, 34, 55–58. [Google Scholar]
- Lyon, S.B.; Bingham, R.; Mills, D.J. Advances in corrosion protection by organic coatings: What we know and what we would like to know. Prog. Org. Coat. 2017, 102, 2–7. [Google Scholar] [CrossRef]
- Mingo, B.; Arrabal, R.; Mohedano, M.; Llamazares, Y.; Matykina, E.; Yerokhin, A.; Pardo, A. Influence of sealing post-treatments on the corrosion resistance of PEO coated AZ91 magnesium alloy. Appl. Surf. Sci. 2018, 433, 653–667. [Google Scholar] [CrossRef]
- Pan, Z.; Sun, R.; Zhu, S.; Kang, Y.; Huang, B.; Zhu, L. The synthesis, characterization and properties of silicone adhesion promoters for addition-cure silicone rubber. J. Adhes. Sci. Technol. 2018, 32, 1517–1530. [Google Scholar] [CrossRef]
- Wu, K.H.; Chao, C.M.; Yeh, T.F.; Chang, T.C. Thermal stability and corrosion resistance of polysiloxane coatings on 2024-T3 and 6061-T6 aluminum alloy. Surf. Coat. Technol. 2007, 201, 5782–5788. [Google Scholar] [CrossRef]
- Li, J.; Liang, L.; Liu, X.; Ma, H.; Song, J.; Wei, Y. Experimental Studies on Strengthening and Failure Mechanism for the Metal/Silicone Rubber/Metal Bonding System. Int. J. Appl. Mech. 2018, 10, 1850029. [Google Scholar] [CrossRef]
- Ramezanzadeh, B.; Raeisi, E.; Mahdavian, M. Studying various mixtures of 3-aminopropyltriethoxysilane (APS) and tetraethylorthosilicate (TEOS) silanes on the corrosion resistance of mild steel and adhesion properties of epoxy coating. Int. J. Adhes. Adhes. 2015, 63, 166–176. [Google Scholar] [CrossRef]
- Fir, M.; Orel, B.; Vuk, A.Š.; Albanil, L.; Rodošek, M.; Raeisi, E. Corrosion Studies and Interfacial Bonding of Urea/Poly(dimethylsiloxane) Sol/Gel Hydrophobic Coatings on AA 2024 Aluminum Alloy. Langmuir 2007, 23, 5505–5514. [Google Scholar] [CrossRef]
- Šurca Vuk, A.; Fir, M.; Ješe, R.; Vilčnik, A.; Orel, B. Structural studies of sol-gel urea/polydimethylsiloxane barrier coatings and improvement of their corrosion inhibition by addition of various alkoxysilanes. Prog. Org. Coat. 2008, 63, 123–132. [Google Scholar] [CrossRef]
- Ghosh, S.; Greenfeld, I.; Wagner, H.D. CNT coating and anchoring beads enhance interfacial adhesion in fiber composites. Compos. Part A 2023, 167, 107427. [Google Scholar] [CrossRef]
- Yu, C.; Ju, P.; Wan, H.; Chen, L.; Li, H.; Zhou, H.; Chen, J. Tribological properties of the polyacrylate/PTFE coating modified by POSS in the space environment. J. Appl. Polym. Sci. 2019, 137, 48730. [Google Scholar] [CrossRef]
- Pan, K.; Zeng, X.; Li, H.; Lai, X.; Huang, J. Synthesis of an adhesion-enhancing polyhydrosiloxane containing acrylate groups and its cross-linked addition-cure silicone encapsulant. J. Elastomers Plast. 2013, 47, 416–430. [Google Scholar] [CrossRef]
- Deflorian, F.; Rossi, S.; Fedel, M.; Motte, C. Electrochemical investigation of high-performance silane sol-gel films containing clay nanoparticles. Prog. Org. Coat. 2010, 69, 158–166. [Google Scholar] [CrossRef]
- Koželj, M.; Vuk, A.Š.; Jerman, I.; Orel, B. Corrosion protection of Sunselect, a spectrally selective solar absorber coating, by (3-mercaptopropyl)trimethoxysilane. Sol. Energy Mater. Sol. Cells 2009, 93, 1733–1742. [Google Scholar] [CrossRef]
- Zhao, X.; Zang, C.; Sun, Y.; Liu, K.; Wen, Y.; Jiao, Q. Borosiloxane oligomers for improving adhesion of addition-curable liquid silicone rubber with epoxy resin by surface treatment. J. Mater. Sci. 2017, 53, 1167–1177. [Google Scholar] [CrossRef]
- Ghosh, S.; Ganguly, S.; Das, P.; Das, T.K.; Bose, M.; Singha, N.K.; Das, A.K.; Das, N.C. Fabrication of Reduced Graphene Oxide/Silver Nanoparticles Decorated Conductive Cotton Fabric for High Performing Electromagnetic Interference Shielding and Antibacterial Application. Fibers Polym. 2019, 20, 1161–1171. [Google Scholar] [CrossRef]
- Rudawska, A.; Bociąga, E.; Olewnik-Kruszkowska, E. The effect of primers on adhesive properties and strength of adhesive joints made with polyurethane adhesives. J. Adhes. Sci. Technol. 2016, 31, 327–344. [Google Scholar] [CrossRef]
- Toorani, M.; Aliofkhazraei, M.; Mahdavian, M.; Naderi, R. Effective PEO/Silane pretreatment of epoxy coating applied on AZ31B Mg alloy for corrosion protection. Corros. Sci. 2020, 169, 108608. [Google Scholar] [CrossRef]
- Grard, A.; Belec, L.; Perrin, F.X. Characterization and evaluation of primer formulations for bonding silicone rubber to metal. Prog. Org. Coat. 2020, 140, 105513. [Google Scholar] [CrossRef]
- Surca, A.K.; Rauter, A.; Rodošek, M.; Slemenik Perše, L.; Koželj, M.; Orel, B. Modified bis-(3-(3-(3-triethoxysilyl)propyl)thioureido)propyl terminated poly(dimethylsiloxane)/POSS protective coatings on AA 2024. Prog. Org. Coat. 2017, 103, 1–14. [Google Scholar] [CrossRef]
- Koga, H.; Kitaoka, T.; Isogai, A. In situ modification of cellulose paper with amino groups for catalytic applications. J. Mater. Chem. 2011, 21, 9356. [Google Scholar] [CrossRef]
- Picard, L.; Phalip, P.; Fleury, E.; Ganachaud, F. Bonding of silicone rubbers on metal (2) physical chemistry of adhesion. Prog. Org. Coat. 2015, 87, 258–266. [Google Scholar] [CrossRef]
- Osterholtz, F.D.; Pohl, E.R. Kinetics of the hydrolysis and condensation of organofunctional alkoxysilanes: A review. J. Adhes. Sci. Technol. 1992, 6, 127–149. [Google Scholar] [CrossRef]








| Sample | TEOS | TPOS | TBOS | Tensile Shear Strength (MPa) | Failure Mode |
|---|---|---|---|---|---|
| A | 3 | 0 | 0 | 0.843 | Interface damage |
| B | 2 | 1 | 0 | 0.852 | Interface damage |
| C | 0 | 1 | 2 | 0.875 | Interface damage |
| D | 0 | 2 | 1 | 0.912 | Interface damage |
| E | 0 | 0 | 3 | 0.820 | Interface damage |
| F | 0 | 3 | 0 | 0.973 | Interface damage |
| G | 1 | 2 | 0 | 0.974 | Interface damage |
| H | 1 | 2 | 1 | 1.138 | Interface damage |
| I | 1 | 3 | 1 | 1.330 | Interface damage |
| J | 1 | 4 | 1 | 0.937 | Interface damage |
| K | 1 | 5 | 1 | 0.668 | Interface damage |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, T.; Su, Z.; Yan, Y.; Zhu, X.; Qi, F.; Wu, L. The Synergistic Effects of Aminosilane Coupling Agent on the Adhesion Performance of Silane Primer for Silicone Resin Thermal Protection Coating. Polymers 2023, 15, 2361. https://doi.org/10.3390/polym15102361
Pan T, Su Z, Yan Y, Zhu X, Qi F, Wu L. The Synergistic Effects of Aminosilane Coupling Agent on the Adhesion Performance of Silane Primer for Silicone Resin Thermal Protection Coating. Polymers. 2023; 15(10):2361. https://doi.org/10.3390/polym15102361
Chicago/Turabian StylePan, Ting, Zhenhua Su, Yue Yan, Xiaofei Zhu, Fan Qi, and Lianbin Wu. 2023. "The Synergistic Effects of Aminosilane Coupling Agent on the Adhesion Performance of Silane Primer for Silicone Resin Thermal Protection Coating" Polymers 15, no. 10: 2361. https://doi.org/10.3390/polym15102361