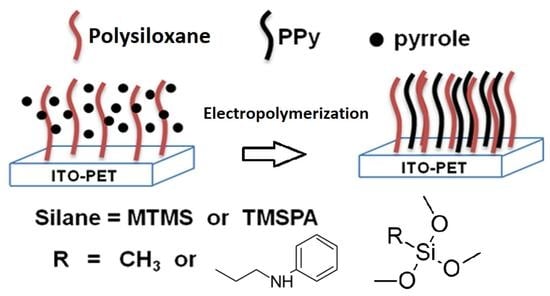
Electrochemical Deposition of Polypyrrole in the Presence of Silanes as Adhesion Promoters
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of the Monomer/Alkoxysilanes Solution for Electropolymerization
2.3. Electrodeposition of PPy Samples
2.4. Characterization
3. Results
3.1. Electrodeposition of PPy/Alkoxysilanes
3.2. Film Morphology
| Sample Label | Alkoxysilanes | Charge Density (mC/cm2) | Thickness (nm) |
|---|---|---|---|
| PPy | None | 397 | 484 |
| PPy–MTMS | MTMS | 213 | 338 |
| PPy–TMSPA | TMSPA | 69 | 36 |
3.3. Spectroscopic Characterization

3.4. Adhesion Test
| Sample | Element Composition (%) | Ratio Si/O | ||||
|---|---|---|---|---|---|---|
| O 1s | C 1s | Si 2p | N 1s | Cl 2p | ||
| PPy | 10.0 | 69.9 | - | 17.5 | 2.6 | - |
| PPy–MTMS | 40.6 | 30.7 | 21.1 | 3.9 | 3.7 | 0.52 |
| PPy–TMSPA | 25.2 | 50.1 | 13.4 | 8.5 | 2.8 | 0.53 |
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hao, L.; Dong, C.Y.; Zhang, L.F.; Zhu, K.M.; Yu, D.M. Polypyrrole Nanomaterials: Structure, Preparation and Application. Polymers 2022, 14, 5139. [Google Scholar] [CrossRef] [PubMed]
- Namsheer, K.; Rout, C.S. Conducting polymers: A comprehensive review on recent advances in synthesis, properties and applications. RSC Adv. 2021, 11, 5659–5697. [Google Scholar] [CrossRef]
- Nautiyal, A.; Qiao, M.Y.; Cook, J.E.; Zhang, X.Y.; Huang, T.S. High performance polypyrrole coating for corrosion protection and biocidal applications. Appl. Surf. Sci. 2018, 427, 922–930. [Google Scholar] [CrossRef]
- Shi, H.; Pan, K.; Dai, M.; Wei, W.; Liu, X.Y.; Li, X.J. A Gallic Acid-Doped Polypyrrole Coating with Anticorrosion and Antibacterial Properties on Magnesium Alloy. ACS Appl. Bio Mater. 2022, 5, 4244–4255. [Google Scholar] [CrossRef]
- Patterson, N.; Xiao, B.; Ignaszak, A. Polypyrrole decorated metal-organic frameworks for supercapacitor devices. RSC Adv. 2020, 10, 20162–20172. [Google Scholar] [CrossRef]
- Gao, F.X.; Zhang, N.; Fang, X.D.; Ma, M.M. Bioinspired Design of Strong, Tough, and Highly Conductive Polyol-Polypyrrole Composites for Flexible Electronics. ACS Appl. Mater. Interfaces 2017, 9, 5692–5698. [Google Scholar] [CrossRef]
- Rabl, H.; Wielend, D.; Tekoglu, S.; Seelajaroen, H.; Neugebauer, H.; Heitzmann, N.; Apaydin, D.H.; Scharber, M.C.; Sariciftci, N.S. Are Polyaniline and Polypyrrole Electrocatalysts for Oxygen (O-2) Reduction to Hydrogen Peroxide (H2O2)? ACS Appl. Energy Mater. 2020, 3, 10611–10618. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.K.; Yu, J.M.; Huang, T.Z.; Sun, M. Recent Advance on Polyaniline or Polypyrrole-Derived Electrocatalysts for Oxygen Reduction Reaction. Polymers 2018, 10, 1397. [Google Scholar] [CrossRef]
- Pina-Beltran, D.U.; Hernandez-Tenorio, C.; Escobedo, C.A.C.; Villanueva-Castaneda, M.; Moreno-Saavedra, H. Electrodeposition and characterization of polypyrrole films on T304 stainless steel. MRS Adv. 2022, 7, 69–72. [Google Scholar] [CrossRef]
- Velhal, N.; Kulkarni, G.; Patil, N.D.; Puri, V. Structural, electrical and microwave properties of conducting polypyrrole thin films: Effect of oxidant. Mater. Res. Express 2018, 5, 106407. [Google Scholar] [CrossRef]
- Cruz-Silva, R.; Amaro, E.; Escamilla, A.; Nicho, M.E.; Sepulveda-Guzman, S.; Arizmendi, L.; Romero-Garcia, J.; Castillon-Barraza, F.F.; Farias, M.H. Biocatalytic synthesis of polypyrrole powder, colloids, and films using horseradish peroxidase. J. Colloid Interface Sci. 2008, 328, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Simon, R.A.; Ricco, A.J.; Wrighton, M.S. Synthesis and characterization of a new surface derivatizing reagent to promote the adhesion of polypyrrole films to N-type silicon photoanodes: N-(3-(trimethoxysilyl)propyl)pyrrole. J. Am. Chem. Soc. 1982, 104, 2031–2034. [Google Scholar] [CrossRef]
- Sougueh, C.M.; Lakard, S.; Husson, J.; Contal, E.; Monney, S.; Moutarlier, V.; Magnenet, C.; Lakard, B. Influence of pre-grafted pyrrole-based silane on the electrodeposition and chemical properties of polypyrrole films. Synth. Met. 2018, 246, 220–229. [Google Scholar] [CrossRef]
- Inoue, A.; Yuk, H.; Lu, B.Y.; Zhao, X.H. Strong adhesion of wet conducting polymers on diverse substrates. Sci. Adv. 2020, 6, eaay5394. [Google Scholar] [CrossRef]
- Adhikari, A.; Radhakrishnan, S.; Vijayan, M. Effect of the surface roughness of conducting polypyrrole thin-film electrodes on the electrocatalytic reduction of nitrobenzene. J. Appl. Polym. Sci. 2012, 125, 1875–1881. [Google Scholar] [CrossRef]
- Kim, S.; Jang, L.K.; Park, H.S.; Lee, J.Y. Electrochemical deposition of conductive and adhesive polypyrrole-dopamine films. Sci. Rep. 2016, 6, 30475. [Google Scholar] [CrossRef]
- Chen, Y.H.; Lai, Y.H.; Wu, P.H.; Chen, L.S.; Lin, Y.S.; Chen, C.M. Mutual intercropping-inspired co-silanization to graft well-oriented organosilane as adhesion promotion nanolayer for flexible conductors. J. Ind. Eng. Chem. 2020, 83, 90–99. [Google Scholar] [CrossRef]
- Castro-Beltran, A.; Dominguez, C.; Bahena-Uribe, D.; Sepulveda-Guzman, S.; Cruz-Silva, R. Effect of non-electroactive additives on the early stage pyrrole electropolymerization on indium tin oxide electrodes. Thin Solid Film. 2014, 566, 23–31. [Google Scholar] [CrossRef]
- Andre, J.S.; Grant, J.; Greyson, E.; Chen, X.Y.; Tucker, C.; Drumright, R.; Mohler, C.; Chen, Z. Molecular Interactions between Amino Silane Adhesion Promoter and Acrylic Polymer Adhesive at Buried Silica Interfaces. Langmuir 2022, 38, 6180–6190. [Google Scholar] [CrossRef]
- Qiao, Y.S.; Shen, L.Z.; Guo, Y.; Liu, J.H.; Meng, S.M. Polypyrrole films prepared on self-assembled silane monolayers and applications. Mater. Technol. 2015, 30, 182–188. [Google Scholar] [CrossRef]
- Liu, T.J.; Sil, M.C.; Chen, C.M. Well-organized organosilane composites for adhesion enhancement of heterojunctions. Compos. Sci. Technol. 2020, 193, 108135. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, X.; Tong, Y.; Sun, J.; Zhao, X.; Liu, X.; Han, X.; Tang, Q.; Liu, Y. Photolithographic Patterning of Polypyrrole on Elastic Polydimethylsiloxane for Flexible and Conformal Organic Electronics. IEEE Electron Device Lett. 2023, 44, 76–79. [Google Scholar] [CrossRef]
- Benzaoui, K.; Ales, A.; Mekki, A.; Zaoui, A.; Bouaouina, B.; Singh, A.; Mehelli, O.; Derradji, M. Electromagnetic interference shielding effectiveness of polypyrrole-silver nanocomposite films on silane-modified flexible sheet. High Perform. Polym. 2022, 34, 310–320. [Google Scholar] [CrossRef]
- Cruz-Silva, R.; Nicho, M.E.; Resendiz, M.C.; Agarwal, V.; Castillon, F.F.; Farias, M.H. Electrochemical polymerization of an aniline-terminated self-assembled monolayer on indium tin oxide electrodes and its effect on polyaniline electrodeposition. Thin Solid Film. 2008, 516, 4793–4802. [Google Scholar] [CrossRef]
- Kohn, W.; Sham, L.J. Self-Consistent Equations Including Exchange and Correlation Effects. Phys. Rev. 1965, 140, A1133–A1138. [Google Scholar] [CrossRef]
- Klamt, A.; Schuurmann, G. COSMO—A new approach to dielectric screening in solvents with explicit expresions for the screening energy and its gradient. J. Chem. Soc.-Perkin Trans. 1993, 2, 799–805. [Google Scholar] [CrossRef]
- Apra, E.; Bylaska, E.J.; de Jong, W.A.; Govind, N.; Kowalski, K.; Straatsma, T.P.; Valiev, M.; van Dam, H.J.J.; Alexeev, Y.; Anchell, J.; et al. NWChem: Past, present, and future. J. Chem. Phys. 2020, 152, 184102. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F.W. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. Model. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Osterholtz, F.D.; Pohl, E.R. Kinetics of the hydrolysis and condensationi of organofunctional alkoxysilanes—A review. J. Adhes. Sci. Technol. 1992, 6, 127–149. [Google Scholar] [CrossRef]
- Cossement, D.; Plumier, F.; Delhalle, J.; Hevesi, L.; Mekhalif, Z. Electrochemical deposition of polypyrrole films on organosilane-modified ITO substrates. Synth. Met. 2003, 138, 529–536. [Google Scholar] [CrossRef]
- Pourhashem, S.; Vaezi, M.R.; Rashidi, A.; Bagherzadeh, M.R. Distinctive roles of silane coupling agents on the corrosion inhibition performance of graphene oxide in epoxy coatings. Prog. Org. Coat. 2017, 111, 47–56. [Google Scholar] [CrossRef]
- Flamini, D.O.; Trueba, M.; Trasatti, S.P. Aniline-based silane as a primer for corrosion inhibition of aluminium. Prog. Org. Coat. 2012, 74, 302–310. [Google Scholar] [CrossRef]
- Syugaev, A.V.; Lyalina, N.V.; Maratkanova, A.N.; Kurenya, A.G. Effect of carbon nanotubes and finely-dispersed graphite particles on electrodeposition of polypyrrole. Synth. Met. 2020, 262, 116350. [Google Scholar] [CrossRef]
- Chen, Y.; Li, J.J.; Zhang, X.; Xu, H. Effects of transition metal ions on the electrochemical performance of polypyrrole electrode. J. Mater. Sci.-Mater. Electron. 2018, 29, 11020–11029. [Google Scholar] [CrossRef]
- Peshoria, S.; Narula, A.K. One-pot synthesis of porphyrin@polypyrrole hybrid and its application as an electrochemical sensor. Mater. Sci. Eng. B-Adv. Funct. Solid-State Mater. 2018, 229, 53–58. [Google Scholar] [CrossRef]
- Firat, E.; Peksoz, A. Efficiently two-stage synthesis and characterization of CuSe/Polypyrrole composite thin films. J. Alloys Compd. 2017, 727, 177–184. [Google Scholar] [CrossRef]
- Zhang, M.M.; Nautiyal, A.; Du, H.S.; Li, J.H.; Liu, Z.Q.; Zhang, X.Y.; Wang, R.G. Polypyrrole film based flexible supercapacitor: Mechanistic insight into influence of acid dopants on electrochemical performance. Electrochim. Acta 2020, 357, 136877. [Google Scholar] [CrossRef]
- Menkuer, M.; Ozkazanc, H. Electrodeposition of polypyrrole on copper surfaces in OXA-DBSA mix electrolyte and their corrosion behaviour. Prog. Org. Coat. 2019, 130, 149–157. [Google Scholar] [CrossRef]
- Beak, K.; Choi, M.; Kim, D.H.; Yu, Y.; Theerthagiri, J.; Al-Mohaimeed, A.M.; Kim, Y.; Jung, H.J.; Choi, M.Y. Silane-treated BaTiO3 ceramic powders for multilayer ceramic capacitor with enhanced dielectric properties. Chemosphere 2022, 286, 131734. [Google Scholar] [CrossRef]
- Briggs, D. New developments in polymer surface analysis. Polymer 1984, 25, 1379–1391. [Google Scholar] [CrossRef]
- Park, B.D.; Wi, S.G.; Lee, K.H.; Singh, A.P.; Yoon, T.H.; Kim, Y.S. X-ray photoelectron spectroscopy of rice husk surface modified with maleated polypropylene and silane. Biomass Bioenergy 2004, 27, 353–363. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castro-Beltran, A.; Alvarado-Beltran, C.G.; Lara-Sanchez, J.F.; de la Cruz, W.; Castillon-Barraza, F.F.; Cruz-Silva, R. Electrochemical Deposition of Polypyrrole in the Presence of Silanes as Adhesion Promoters. Polymers 2023, 15, 2354. https://doi.org/10.3390/polym15102354
Castro-Beltran A, Alvarado-Beltran CG, Lara-Sanchez JF, de la Cruz W, Castillon-Barraza FF, Cruz-Silva R. Electrochemical Deposition of Polypyrrole in the Presence of Silanes as Adhesion Promoters. Polymers. 2023; 15(10):2354. https://doi.org/10.3390/polym15102354
Chicago/Turabian StyleCastro-Beltran, Andres, Clemente G. Alvarado-Beltran, Jesus F. Lara-Sanchez, Wencel de la Cruz, Felipe F. Castillon-Barraza, and Rodolfo Cruz-Silva. 2023. "Electrochemical Deposition of Polypyrrole in the Presence of Silanes as Adhesion Promoters" Polymers 15, no. 10: 2354. https://doi.org/10.3390/polym15102354