Highly Stable Docetaxel-Loaded Nanoparticles Based on Poly(D,L-lactide)-b-Poly(ethylene glycol) for Cancer Treatment: Preparation, Characterization, and In Vitro Cytotoxicity Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Block Copolymers
2.3. Characterization of Block Copolymers
2.4. Preparation of P(D,L)LAn-b-PEG113 Nanoparticles
2.5. Characterization of P(D,L)LAn-b-PEG113 Nanoparticles
2.5.1. Dynamic Light Scattering (DLS)
2.5.2. Electrophoretic Light Scattering (ELS)
2.5.3. Transmission Electron Microscopy (TEM)
2.5.4. High-Performance Liquid Chromatography (HPLC)
2.5.5. In Vitro Drug Release Profiles
2.5.6. Cells and In Vitro Cytotoxicity Assay
2.5.7. Freeze-Drying of P(D,L)LAn-b-PEG113 Nanoparticles
2.6. Statistical Analysis
3. Results and Discussions
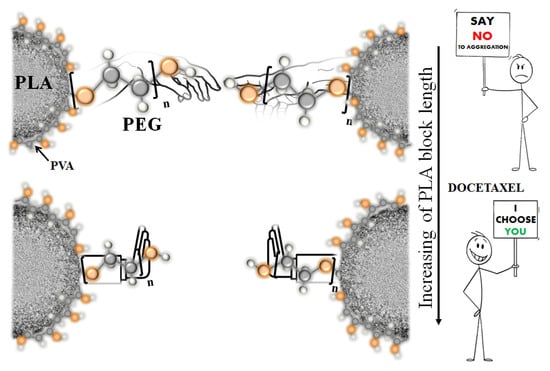
3.1. Characterization of Drug-Free P(D,L)LAn-b-PEG113 Nanoparticles
3.2. Characterization of Docetaxel-Loaded P(D,L)LAn-b-PEG113 Nanoparticles
3.3. Docetaxel Release Studies
3.4. In Vitro Cytotoxicity Studies
3.5. Freeze-Drying of Docetaxel-Loaded P(D,L)LAn-b-PEG113 Particles
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Selmani, A.; Kovačević, K. Nanoparticles: From synthesis to applications and beyond. Adv. Colloid Interface Sci. 2022, 303, 102640. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Gong, J. Strategies for liposome drug delivery systems to improve tumor treatment efficacy. AAPS PharmSciTech 2021, 23, 27. [Google Scholar] [CrossRef] [PubMed]
- Yaroslavov, A.A.; Efimova, A.A.; Sybachin, A.V.; Chvalun, S.N.; Kulebyakina, A.I.; Kozlova, E.V. Biodegradable multi-liposomal containers. RSC Adv. 2015, 5, 31460–31464. [Google Scholar] [CrossRef]
- Yaroslavov, A.A.; Efimova, A.A.; Rudenskaya, G.N.; Melik-Nubarov, N.S.; Grozdova, I.D.; Ezhov, A.A.; Chvalun, S.N.; Kulebyakina, A.I.; Razuvaeva, E.V. An electrostatic conjugate composed of liposomes, polylysine and a polylactide micelle: A biodegradability–cytotoxicity relationship. Mendeleev Commun. 2017, 27, 299–301. [Google Scholar] [CrossRef]
- Sedush, N.G.; Kadina, Y.A.; Razuvaeva, E.V.; Puchkov, A.A.; Shirokova, E.M.; Gomzyak, V.I.; Kalinin, K.T.; Kulebyakina, A.I.; Chvalun, S.N. Nanoformulations of drugs based on biodegradable lactide copolymers with various molecular structures and architectures. Nanobiotechnol. Rep. 2021, 16, 421–438. [Google Scholar] [CrossRef]
- Ibarra-Sánchez, L.; Gámez-Méndez, A.; Martínez-Ruiz, M.; Nájera-Martínez, E.F.; Morales-Flores, B.A.; Melchor-Martínez, E.M.; Sosa-Hernández, J.E.; Parra-Saldívar, R.; Iqbal, H.M. Nanostructures for drug delivery in respiratory diseases therapeutics: Revision of current trends and its comparative analysis. J. Drug Deliv. Sci. Technol. 2022, 70, 103219. [Google Scholar] [CrossRef]
- Brzeziński, M.; Kost, B.; Gonciarz, W.; Krupa, A.; Socka, M.; Rogala, M. Nanocarriers based on block copolymers of l-proline and lactide: The effect of core crosslinking versus its pH-sensitivity on their cellular uptake. Eur. Polym. J. 2021, 156, 110572. [Google Scholar] [CrossRef]
- Sathe, R.Y.; Bharatam, P.V. Drug-dendrimer complexes and conjugates: Detailed furtherance through theory and experiments. Adv. Colloid Interface Sci. 2022, 303, 102639. [Google Scholar] [CrossRef]
- De Oliveira Costa, R.; Coutinho, J.P.; Santos, R.L.S.R. Use of mixture design to optimize nanofabrication of dithiocarbazate–loaded polylactic acid nanoparticles. J. Appl. Polym. Sci. 2022, 139, 51504. [Google Scholar] [CrossRef]
- Ye, L.; Yao, Q.; Xu, F.; He, L.; Ding, J.; Xiao, R.; Ding, L.; Luo, B. Preparation and antitumor activity of triphenylphosphine-based mitochondrial targeting polylactic acid nanoparticles loaded with 7-hydroxyl coumarin. J. Biomater. Appl. 2022, 36, 1064–1075. [Google Scholar] [CrossRef]
- Brzeziński, M.; Wedepohl, S.; Kost, B.; Calderón, M. Nanoparticles from supramolecular polylactides overcome drug resistance of cancer cells. Eur. Polym. J. 2018, 109, 117–123. [Google Scholar] [CrossRef]
- Snejdrova, E.; Loskot, J.; Martiska, J.; Soukup, T.; Prokes, L.; Frolov, V.; Kucera, T. Rifampicin-loaded PLGA nanoparticles for local treatment of musculoskeletal infections: Formulation and characterization. J. Drug Deliv. Sci. Technol. 2022, 73, 103435. [Google Scholar] [CrossRef]
- Razuvaeva, E.V.; Kalinin, K.T.; Sedush, N.G.; Nazarov, A.A.; Volkov, D.S.; Chvalun, S.N. Structure and cytotoxicity of biodegradable poly(D,L-lactide-co-glycolide) nanoparticles loaded with oxaliplatin. Mendeleev Commun. 2021, 31, 512–514. [Google Scholar] [CrossRef]
- Poltavets, Y.I.; Zhirnik, A.S.; Zavarzina, V.V.; Semochkina, Y.P.; Shuvatova, V.G.; Krasheninnikova, A.A.; Aleshin, S.V.; Dronov, D.O.; Vorontsov, E.A.; Balabanyan, V.Y.; et al. In vitro anticancer activity of folate-modified docetaxel-loaded PLGA nanoparticles against drug-sensitive and multidrug-resistant cancer cells. Cancer Nanotechnol. 2019, 10, 2. [Google Scholar] [CrossRef]
- Patel, P.; Raval, M.; Manvar, A.; Airao, V.; Bhatt, V.; Shah, P. Lung cancer targeting efficiency of Silibinin loaded Poly Caprolactone/Pluronic F68 Inhalable nanoparticles: In vitro and In vitro study. PLoS ONE 2022, 17, e0267257. [Google Scholar] [CrossRef] [PubMed]
- Ábrahám, Á.; Gyalai, G. Improvement of the drug encapsulation into biodegradable polyester nanocarriers by blending of poly(lactic-co-glycolic acid) and polycaprolactone. Express Polym. Lett. 2022, 16, 960–977. [Google Scholar] [CrossRef]
- Carvalho, J.A.; da Silva Abreu, A.; Tedesco, A.; Junior, M.B.; Simioni, A.R. Functionalized photosensitive gelatin nanoparticles for drug delivery application. J. Biomater. Sci. Polym. Ed. 2019, 30, 508–525. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, K.; Peng, X.; Zhang, L. Chitosan-based drug delivery systems: Current strategic design and potential application in human hard tissue repair. Eur. Polym. J. 2022, 166, 110979. [Google Scholar] [CrossRef]
- Razuvaeva, E.; Sedush, N.; Shirokova, E.; Moskvichev, S.; Streltsov, D.; Chvalun, S. Effect of preparation conditions on the size of nanoparticles based on poly(D,L-lactide-co-glycolide) synthesized with bismuth subsalicylate. Colloids Surfaces A Physicochem. Eng. Asp. 2022, 648, 129198. [Google Scholar] [CrossRef]
- Ruiz, E.; Orozco, V.H.; Hoyos, L.M.; Giraldo, L.F. Study of sonication parameters on PLA nanoparticles preparation by simple emulsion-evaporation solvent technique. Eur. Polym. J. 2022, 173, 111307. [Google Scholar] [CrossRef]
- Kost, B.; Basko, M.; Bednarek, M.; Socka, M.; Kopka, B.; Łapienis, G.; Biela, T.; Kubisa, P.; Brzeziński, M. The influence of the functional end groups on the properties of polylactide-based materials. Prog. Polym. Sci. 2022, 130, 101556. [Google Scholar] [CrossRef]
- Sahin, A.; Esendagli, G.; Yerlikaya, F.; Caban-Toktas, S.; Yoyen-Ermis, D.; Horzum, U.; Aktas, Y.; Khan, M.; Couvreur, P.; Capan, Y. A small variation in average particles size of PLGA nanoparticles prepared by nanoprecipitation leads to considerable change in nanoparticles’ characteristics and efficiency of intracellular delivery. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1657–1664. [Google Scholar] [CrossRef]
- Baoum, A.; Dhillon, N.; Buch, S.; Berkland, C. Cationic surface modification of PLG nanoparticles offers sustained gene delivery to pulmonary epithelial cells. J. Pharm. Sci. 2010, 99, 2413–2422. [Google Scholar] [CrossRef] [PubMed]
- Parveen, S.; Sahoo, S.K. Long circulating chitosan/PEG blended PLGA nanoparticle for tumor drug delivery. Eur. J. Pharmacol. 2011, 670, 372–383. [Google Scholar] [CrossRef] [PubMed]
- Tyrrell, Z.L.; Shen, Y.; Radosz, M. Fabrication of micellar nanoparticles for drug delivery through the self-assembly of block copolymers. Prog. Polym. Sci. 2010, 35, 1128–1143. [Google Scholar] [CrossRef]
- Miyata, K.; Christie, R.J.; Kataoka, K. Polymeric micelles for nano-scale drug delivery. React. Funct. Polym. 2011, 73, 227–234. [Google Scholar] [CrossRef]
- Gong, C.; Xie, Y.; Wu, Q.; Wang, Y.; Deng, S.; Xiong, D.; Liu, L.; Xiang, M.; Qian, Z.; Wei, Y. Improving anti-tumor activity with polymeric micelles entrapping paclitaxel in pulmonary carcinoma. Nanoscale 2012, 4, 6004–6017. [Google Scholar] [CrossRef] [PubMed]
- Razak, S.A.A.; Gazzali, A.M.; Fisol, F.A.; Abdulbaqi, I.M.; Parumasivam, T.; Mohtar, N.; Wahab, H.A. Advances in nanocarriers for effective delivery of docetaxel in the treatment of lung cancer: An overview. Cancers 2021, 13, 400. [Google Scholar] [CrossRef]
- Kadina, Y.A.; Razuvaeva, E.V.; Streltsov, D.R.; Sedush, N.G.; Shtykova, E.V.; Kulebyakina, A.I.; Puchkov, A.A.; Volkov, D.S.; Nazarov, A.A.; Chvalun, S.N. Poly(ethylene glycol)-b-poly(D,L-lactide) nanoparticles as potential carriers for anticancer drug oxaliplatin. Molecules 2021, 26, 602. [Google Scholar] [CrossRef]
- Muddineti, O.S.; Shah, A.; Rompicharla, S.V.K.; Ghosh, B.; Biswas, S. Cholesterol-grafted chitosan micelles as a nanocarrier system for drug-siRNA co-delivery to the lung cancer cells. Int. J. Biol. Macromol. 2018, 118, 857–863. [Google Scholar] [CrossRef]
- Kim, G.; Piao, C.; Oh, J.; Lee, M. Self-assembled polymeric micelles for combined delivery of anti-inflammatory gene and drug to the lungs by inhalation. Nanoscale 2018, 10, 8503–8514. [Google Scholar] [CrossRef] [PubMed]
- Gong, F.; Wang, R.; Zhu, Z.; Duan, J.; Teng, X.; Cui, Z.-K. Drug-interactive mPEG-b-PLA-Phe(Boc) micelles enhance the tolerance and anti-tumor efficacy of docetaxel. Drug Deliv. 2020, 27, 238–247. [Google Scholar] [CrossRef]
- Wan, X.; Min, Y.; Bludau, H.; Keith, A.; Sheiko, S.S.; Jordan, R.; Wang, A.Z.; Sokolsky-Papkov, M.; Kabanov, A.V. Drug combination synergy in worm-like polymeric micelles improves treatment outcome for small cell and non-small cell lung cancer. ACS Nano 2018, 12, 2426–2439. [Google Scholar] [CrossRef]
- Khodaverdi, E.; Tayarani-Najaran, Z. Docetaxel-loaded mixed micelles and polymersomes composed of poly(caprolactone)-poly(ethylene glycol) (PEG-PCL) and poly(lactic acid)-poly(ethylene glycol) (PEG-PLA): Preparation and in-vitro characterization. Int. J. Pharm Res. 2019, 18, 142–155. [Google Scholar]
- Zhang, L.; Tan, L.W.; Chen, L.; Chen, X.; Long, C.; Peng, J.; Qian, Z. A simple method to improve the stability of docetaxel micelles. Sci. Rep. 2016, 6, 36957. [Google Scholar] [CrossRef]
- Yang, M.; Ding, Y.; Zhang, L.; Qian, X.; Jiang, X.; Liu, B. Novel thermosensitive polymeric micelles for docetaxel delivery. J. Biomed. Mater. Res. A 2007, 81, 847–857. [Google Scholar] [CrossRef] [PubMed]
- Mikhail, A.S.; Eetezadi, S. Multicellular tumor spheroids for evaluation of cytotoxicity and tumor growth inhibitory effects of nanomedicines in vitro: A comparison of docetaxel-loaded block copolymer micelles and Taxotere®. PLoS ONE 2013, 8, e62630. [Google Scholar] [CrossRef]
- Tang, X.; Zhou, S.; Tao, X.; Wang, J.; Wang, F.; Liang, Y. Targeted delivery of docetaxel via Pi-Pi stacking stabilized dendritic polymeric micelles for enhanced therapy of liver cancer. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 75, 1042–1048. [Google Scholar] [CrossRef]
- Ghamkhari, A.; Sarvari, R.; Ghorbani, M.; Hamishehkar, H. Novel thermoresponsive star-liked nanomicelles for targeting of anticancer agent. Eur. Polym. J. 2018, 107, 143–154. [Google Scholar] [CrossRef]
- Lang, T.; Dong, X.; Zheng, Z.; Liu, Y.; Wang, G.; Yin, Q.; Li, Y. Tumor microenvironment-responsive docetaxel-loaded micelle combats metastatic breast cancer. Sci. Bull. 2019, 64, 91–100. [Google Scholar] [CrossRef]
- Tan, L.; Peng, J.; Zhao, Q.; Zhang, L.; Tang, X.; Chen, L.; Lei, M.; Qian, Z. A novel MPEG-PDLLA-PLL copolymer for docetaxel delivery in breast cancer therapy. Theranostics 2017, 7, 2652–2672. [Google Scholar] [CrossRef]
- Shi, J.; Zhang, J.; Shen, Y.; Tang, L.; Zhao, J.; Tu, J.; Tian, Y.; Feng, Y. Arginine-stabilized mPEG-PDLLA (50/50) polymeric micelles of docetaxel by electrostatic mechanism for tumor-targeted delivery. Drug Deliv. 2015, 22, 168–181. [Google Scholar] [CrossRef]
- Shutkov, I.A.; Okulova, Y.N.; Tyurin, V.Y.; Sokolova, E.V.; Babkov, D.A.; Spasov, A.A.; Gracheva, Y.A.; Schmidt, C.; Kirsanov, K.I.; Shtil, A.A.; et al. Ru(III) complexes with lonidamine-modified ligands. Int. J. Mol. Sci. 2021, 22, 13468. [Google Scholar] [CrossRef] [PubMed]
- Tomaszewska, E.; Soliwoda, K.; Kadziola, K.; Tkacz-Szczesna, B.; Celichowski, G.; Cichomski, M.; Szmaja, W.; Grobelny, J. Detection limits of DLS and UV-Vis spectroscopy in characterization of polydisperse nanoparticles colloids. J. Nanomater. 2013, 2013, 313081. [Google Scholar] [CrossRef]
- Ghasemi, R.; Abdollahi, M.; Zadeh, E.E.; Khodabakhshi, K.; Badeli, A.; Bagheri, H.; Hosseinkhani, S. mPEG-PLA and PLA-PEG-PLA nanoparticles as new carriers for delivery of recombinant human Growth Hormone (rhGH). Sci. Rep. 2018, 8, 9854. [Google Scholar] [CrossRef] [PubMed]
- Mora-Huertas, C.E.; Fessi, H.; Elaissari, A. Influence of process and formulation parameters on the formation of submicron particles by solvent displacement and emulsification–diffusion methods: Critical comparison. Adv. Colloid Interface Sci. 2011, 163, 90–122. [Google Scholar] [CrossRef] [PubMed]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef]
- Kuznetsova, E.V.; Kuznetsov, N.M.; Kalinin, K.T.; Lebedev-Stepanov, P.V.; Novikov, A.A.; Chvalun, S.N. The role of integrated approach in the determination of nanoparticle sizes in dispersions. Colloid J. 2022, 84, 704–714. [Google Scholar] [CrossRef]
- Yu, J.-J.; Jeong, Y.-I.; Shim, Y.-H.; Lim, G.-T. Preparation of core-shell type nanoparticles of diblock copolymers of poly(L-lactide)/poly(ethylene glycol) and their characterizationin vitro. J. Appl. Polym. Sci. 2002, 85, 2625–2634. [Google Scholar] [CrossRef]
- Razuvaeva, E.V.; Kulebyakina, A.I. Effect of composition and molecular structure of poly(L-lactic acid)/poly(ethylene oxide) block copolymers on micellar morphology in aqueous solution. Langmuir 2018, 34, 15470–15482. [Google Scholar] [CrossRef]
- Desyatskova, A.M.; Kuznetsova, E.V.; Puchkova, Y.A.; Yastremsky, E.V.; Bakirov, A.V.; Dmitryakov, P.V.; Buzin, A.I.; Chvalun, S.N. Effect of stereocomplex formation between enantiomeric poly(L,L-lactide) and poly(D,D-lactide) blocks on self-organization of amphiphilic poly(lactide)-block-poly(ethylene oxide) copolymers in dilute aqueous solution. Mendeleev Commun. 2023, 33, 86–89. [Google Scholar] [CrossRef]
- Xu, R.; Winnik, M.A. Light-scattering study of the association behavior of styrene-ethylene oxide block copolymers in aqueous solution. Macromolecules 1991, 24, 87–93. [Google Scholar] [CrossRef]
- Bronstein, L.M.; Chernyshov, D.M.; Timofeeva, G.I.; Dubrovina, L.V.; Valetsky, P.M.; Khokhlov, A.R. Polystyrene-block-poly(ethylene oxide) micelles in aqueous solution. Langmuir 1999, 15, 6195–6200. [Google Scholar] [CrossRef]
- Ho, D.L.; Hammouda, B.; Kline, S.R. Clustering of poly(ethylene oxide) in water revisited. J. Polym. Sci. Part B Polym. Phys. 2003, 41, 135–138. [Google Scholar] [CrossRef]
- Polverari, M.; van de Ven, T.G.M. Dilute aqueous poly(ethylene oxide) solutions: Clusters and single molecules in thermodynamic equilibrium. J. Phys. Chem. 1996, 100, 13687–13695. [Google Scholar] [CrossRef]
- Shetty, A.M.; Solomon, M.J. Aggregation in dilute solutions of high molar mass poly(ethylene) oxide and its effect on polymer turbulent drag reduction. Polymer 2009, 50, 261–270. [Google Scholar] [CrossRef]
- Hammouda, B.; Ho, D.; Kline, S. SANS from poly(ethylene oxide)/water systems. Macromolecules 2002, 35, 8578–8585. [Google Scholar] [CrossRef]
- Hammouda, B.; Ho, D.L.; Kline, S. Insight into clustering in poly(ethylene oxide) solutions. Macromolecules 2004, 37, 6932–6937. [Google Scholar] [CrossRef]
- Devanand, K.; Selser, J.C. Polyethylene oxide does not necessarily aggregate in water. Nature 1990, 343, 739–741. [Google Scholar] [CrossRef]
- Ma, C.; Pan, P.; Shan, G.; Bao, Y.; Fujita, M.; Maeda, M. Core−shell structure, biodegradation, and drug release behavior of poly(lactic acid)/poly(ethylene glycol) block copolymer micelles tuned by macromolecular stereostructure. Langmuir 2015, 31, 1527–1536. [Google Scholar] [CrossRef]
- Fonseca, C.; Simões, S.; Gaspar, R. Paclitaxel-loaded PLGA nanoparticles: Preparation, physicochemical characterization and in vitro anti-tumoral activity. J. Control. Release 2002, 83, 273–286. [Google Scholar] [CrossRef] [PubMed]
- Chu, B.Y.; Zhang, L.; Qu, Y.; Chen, X.; Peng, J.; Huang, Y.; Qian, Z. Synthesis, characterization and drug loading property of monomethoxy-poly(ethylene glycol)-poly(ε-caprolactone)-poly(D,L-lactide) (MPEG-PCLA) copolymers. Sci. Rep. 2016, 6, 34069. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Tao, W.; Wang, Z.; Zhang, X.; Zhu, H.; Wu, Y.; Gao, Y.; Liu, K.; Jiang, Y.; Huang, L.; et al. Docetaxel-loaded nanoparticles of dendritic amphiphilic block copolymer H40-PLA-b-TPGS for cancer treatment. Part. Part. Syst. Charact. 2015, 32, 112–122. [Google Scholar] [CrossRef]






| Block Copolymer | Mn 1, kDa | Mn 2, kDa | Mw 2, kDa | PDI 2 |
|---|---|---|---|---|
| P(D,L)LA50-b-PEG113 | 8.6 | 10.8 | 13.0 | 1.2 |
| P(D,L)LA180-b-PEG113 | 18.1 | 21.3 | 27.1 | 1.3 |
| P(D,L)LA680-b-PEG113 | 53.8 | 25.7 | 54.7 | 2.1 |
| P(D,L)LA1230-b-PEG113 | 93.5 | 41.7 | 121.5 | 2.9 |
| Lyoprotectant | Concentration, g/L |
|---|---|
| D(-)-mannitol | 1, 5, 10 |
| PVA (30–70 kDa) | 2.5, 5, 12.5, 25 |
| mPEG (2 kDa) | 2.5, 5, 12.5, 25 |
| mPEG (5 kDa) | 2.5, 5, 12.5, 25 |
| PEG (10 kDa) | 2.5, 5, 12.5, 25 |
| Sample | (Dh)0 1, nm | PDI 2 | ζ 3, mV | D 4, nm | sint 5, nm | σ 6, nm |
|---|---|---|---|---|---|---|
| P(D,L)LA50-b-PEG113 | 64 ± 13 * | 0.32 ± 0.04 | −9 ± 2 | 33 ± 14 | 0.9 | 1.1 |
| 310 ± 130 ** | ||||||
| P(D,L)LA180-b-PEG113 | 56 ± 14 * | 0.26 ± 0.02 | −15 ± 4 | 51 ± 20 | 2.0 | 0.5 |
| 320 ± 165 ** | ||||||
| P(D,L)LA680-b-PEG113 | 161 ± 1 | 0.11 ± 0.02 | −13 ± 3 | 108 ± 30 | 3.6 | 0.3 |
| P(D,L)LA1230-b-PEG113 | 212 ± 1 | 0.07 ± 0.02 | −12 ± 5 | 142 ± 40 | 5.0 | 0.2 |
| Sample | (Dh)0 1, nm | PDI 2 | ζ 3, mV | D 4, nm | DLC 5, wt% | EE 6, % |
|---|---|---|---|---|---|---|
| P(D,L)LA680-b-PEG113 | 175 ± 1 | 0.08 ± 0.02 | −9 ± 6 | 118 ± 6 | 0.5 ± 0.1 | 10.1 ± 2.0 |
| P(D,L)LA1230-b-PEG113 | 210 ± 2 | 0.09 ± 0.03 | −15 ± 4 | 129 ± 24 | 1.2 ± 0.3 | 24.3 ± 5.2 |
| Sample | IC50, nM | k 1 | |||
|---|---|---|---|---|---|
| MCF7 | HCT116 | A549 | WI38 | ||
| DTX | 3.6 ± 1.0 | 1.5 ± 0.1 | 3.6 ± 0.1 | 4.3 ± 0.3 | 1.2 ± 0.1 |
| P(D,L)LA680-b-PEG113 + DTX | 4.6 ± 1.0 | 1.6 ± 0.3 | 2.9 ± 0.7 | 4.3 ± 0.9 | 1.4 ± 0.4 |
| P(D,L)LA1230-b-PEG113 + DTX | 12.2 ± 0.3 | 5.7 ± 0.1 | 4.9 ± 0.8 | 14.5 ± 2.8 | 3.0 ± 0.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuznetsova, E.V.; Sedush, N.G.; Puchkova, Y.A.; Aleshin, S.V.; Yastremsky, E.V.; Nazarov, A.A.; Chvalun, S.N. Highly Stable Docetaxel-Loaded Nanoparticles Based on Poly(D,L-lactide)-b-Poly(ethylene glycol) for Cancer Treatment: Preparation, Characterization, and In Vitro Cytotoxicity Studies. Polymers 2023, 15, 2296. https://doi.org/10.3390/polym15102296
Kuznetsova EV, Sedush NG, Puchkova YA, Aleshin SV, Yastremsky EV, Nazarov AA, Chvalun SN. Highly Stable Docetaxel-Loaded Nanoparticles Based on Poly(D,L-lactide)-b-Poly(ethylene glycol) for Cancer Treatment: Preparation, Characterization, and In Vitro Cytotoxicity Studies. Polymers. 2023; 15(10):2296. https://doi.org/10.3390/polym15102296
Chicago/Turabian StyleKuznetsova, Ekaterina V., Nikita G. Sedush, Yulia A. Puchkova, Sergei V. Aleshin, Evgeny V. Yastremsky, Alexey A. Nazarov, and Sergei N. Chvalun. 2023. "Highly Stable Docetaxel-Loaded Nanoparticles Based on Poly(D,L-lactide)-b-Poly(ethylene glycol) for Cancer Treatment: Preparation, Characterization, and In Vitro Cytotoxicity Studies" Polymers 15, no. 10: 2296. https://doi.org/10.3390/polym15102296