A Comparative Study on the Aerobic Biodegradation of the Biopolymer Blends of Poly(butylene succinate), Poly(butylene adipate terephthalate) and Poly(lactic acid)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. The Preparation and Processing of the Biopolymer Blends
2.3. Biodegradation Testing
2.4. The Analytical Characterization
3. Results and Discussion
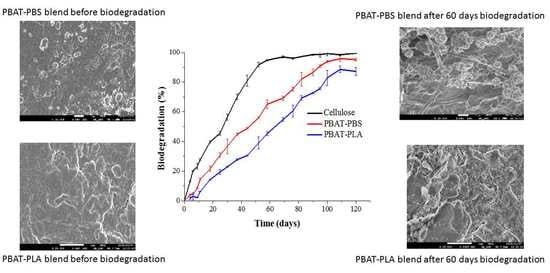
3.1. Biodegradation Stimulated to Industrial Composting Conditions
3.2. FT-IR Spectroscopy
3.3. TGA Analyses
3.4. The DSC Analyses
3.5. The XRD Analyses
3.6. SEM Analyses
3.7. Biodegradation under Home Composting Conditions
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Monya Vermaak, C. Plastics|SA Releases 2015 Plastics Recycling Figures; Plastics|SA: Cape Town, South Africa, 2016; pp. 1–3. [Google Scholar]
- Muniyasamy, S.; Reddy, M.M.; Misra, M.; Mohanty, A. Biodegradable green composites from bioethanol co-product and poly(butylene adipate-co-terephthalate). Ind. Crops Prod. 2013, 43, 812–819. [Google Scholar] [CrossRef]
- Verma, D.; Fortunati, E. Biobased and biodegradable plastics. Handb. Ecomater. 2019, 4, 2955–2976. [Google Scholar] [CrossRef]
- Haider, T.P.; Völker, C.; Kramm, J.; Landfester, K.; Wurm, F.R. Plastics of the Future? The Impact of Biodegradable Polymers on the Environment and on Society. Angew. Chemie Int. Ed. 2019, 58, 50–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palsikowski, P.A.; Kuchnier, C.N.; Pinheiro, I.F.; Morales, A.R. Biodegradation in Soil of PLA/PBAT Blends Compatibilized with Chain Extender. J. Polym. Environ. 2018, 26, 330–341. [Google Scholar] [CrossRef]
- Wang, H.; Wei, D.; Zheng, A.; Xiao, H. Soil burial biodegradation of antimicrobial biodegradable PBAT films. Polym. Degrad. Stab. 2015, 116, 14–22. [Google Scholar] [CrossRef]
- Gowman, A.; Wang, T.; Rodriguez-Uribe, A.; Mohanty, A.K.; Misra, M. Bio-poly (butylene succinate) and its composites with grape pomace: Mechanical performance and thermal properties. ACS Omega 2018, 3, 15205–15216. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, F.V.; Cividanes, L.S.; Gouveia, R.F.; Lona, L.M.F. An overview on properties and applications of poly(butylene adipate-co-terephthalate)–PBAT based composites. Polym. Eng. Sci. 2019, 59, E7–E15. [Google Scholar] [CrossRef] [Green Version]
- De Oliveira, R.R.; De Oliveira, T.A.; Da Silva, L.R.C.; Barbosa, R.; Alves, T.S.; De Carvalho, L.H.; Rodrigues, D.T. Effect of reprocessing cycles on the morphology and mechanical properties of a poly(propylene)/poly(hydroxybutyrate) blend and its nanocomposite. Mater. Res. 2020, 24, 1–11. [Google Scholar] [CrossRef]
- Oliveira, T.A.; Oliveira, R.R.; Barbosa, R.; Azevedo, J.B.; Alves, T.S. Effect of reprocessing cycles on the degradation of PP/PBAT-thermoplastic starch blends. Carbohydr. Polym. 2017, 168, 52–60. [Google Scholar] [CrossRef]
- Fukushima, K.; Abbate, C.; Tabuani, D.; Gennari, M.; Camino, G. Biodegradation of poly(lactic acid) and its nanocomposites. Polym. Degrad. Stab. 2009, 94, 1646–1655. [Google Scholar] [CrossRef]
- Saini, P.; Arora, M.; Kumar, M.N.V.R. Poly(lactic acid) blends in biomedical applications. Adv. Drug Deliv. Rev. 2016, 107, 47–59. [Google Scholar] [CrossRef]
- Al-Itry, R.; Lamnawar, K.; Maazouz, A. Reactive extrusion of PLA, PBAT with a multi-functional epoxide: Physico-chemical and rheological properties. Eur. Polym. J. 2014, 58, 90–102. [Google Scholar] [CrossRef]
- Liu, L.; Yu, J.; Cheng, L.; Yang, X. Biodegradability of poly(butylene succinate) (PBS) composite reinforced with jute fibre. Polym. Degrad. Stab. 2009, 94, 90–94. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, H.J.; Lee, J.W.; Choi, I.G. Biodegradability of bio-flour filled biodegradable poly (butylene succinate) bio-composites in natural and compost soil. Polym. Degrad. Stab. 2006, 91, 1117–1127. [Google Scholar] [CrossRef]
- Palai, B.; Mohanty, S.; Nayak, S.K. A Comparison on Biodegradation Behaviour of Polylactic Acid (PLA) Based Blown Films by Incorporating Thermoplasticized Starch (TPS) and Poly (Butylene Succinate-co-Adipate) (PBSA) Biopolymer in Soil. J. Polym. Environ. 2021, 29, 2772–2788. [Google Scholar] [CrossRef]
- Anstey, A.; Muniyasamy, S.; Reddy, M.M.; Misra, M.; Mohanty, A. Processability and Biodegradability Evaluation of Composites from Poly(butylene succinate) (PBS) Bioplastic and Biofuel Co-products from Ontario. J. Polym. Environ. 2014, 22, 209–218. [Google Scholar] [CrossRef]
- Mohanty, S.; Nayak, S.K. Biodegradable Nanocomposites of Poly(butylene adipate-co-terephthalate) (PBAT) and Organically Modified Layered Silicates. J. Polym. Environ. 2012, 20, 195–207. [Google Scholar] [CrossRef]
- ASTM. ASTM D6400-12; ASTM International: West Conshohocken, PA, USA, 2012. [Google Scholar] [CrossRef]
- Kale, G.; Auras, R.; Singh, S.P.; Narayan, R. Biodegradability of polylactide bottles in real and simulated composting conditions. Polym. Test. 2007, 26, 1049–1061. [Google Scholar] [CrossRef]
- Han, Z.; Ma, H.; Shi, G.; He, L.; Wei, L.; Shi, Q. A review of groundwater contamination near municipal solid waste landfill sites in China. Sci. Total Environ. 2016, 569–570, 1255–1264. [Google Scholar] [CrossRef]
- Bong, C.P.C.; Goh, R.K.Y.; Lim, J.S.; Ho, W.S.; Lee, C.T.; Hashim, H.; Abu Mansor, N.N.; Ho, C.S.; Ramli, A.R.; Takeshi, F. Towards low carbon society in Iskandar Malaysia: Implementation and feasibility of community organic waste composting. J. Environ. Manag. 2017, 203 Pt 2, 679–687. [Google Scholar] [CrossRef]
- Seng, B.; Hirayama, K.; Katayama-Hirayama, K.; Ochiai, S.; Kaneko, H. Scenario analysis of the benefit of municipal organic-waste composting over landfill, Cambodia. J. Environ. Manag. 2013, 114, 216–224. [Google Scholar] [CrossRef]
- Muniyasamy, S.; Ofosu, O.; John, M.J.; Anandjiwala, R.D. Mineralization of poly(lactic acid) (PLA), Poly(3-hydroxybutyrate-co-valerate) (PHBV) and PLA/PHBV blend in compost and soil environments. J. Renew. Mater. 2016, 4, 133–145. [Google Scholar] [CrossRef]
- Bastarrachea, L.; Dhawan, S.; Sablani, S.S.; Mah, J.H.; Kang, D.H.; Zhang, J.; Tang, J. Biodegradable poly(Butylene adipate-co-terephthalate) films incorporated with nisin: Characterization and effectiveness against Listeria innocua. J. Food Sci. 2010, 75, E215–E224. [Google Scholar] [CrossRef]
- Xu, J.; Guo, B.-H. Microbial Succinic Acid, Its Polymer Poly(butylene succinate), and Applications. In Plastics from Bacteria; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Funabashi, M.; Ninomiya, F.; Kunioka, M. Biodegradability evaluation of polymers by ISO 14855-2. Int. J. Mol. Sci. 2009, 10, 3635–3654. [Google Scholar] [CrossRef] [Green Version]
- Touchaleaume, F.; Angellier-Coussy, H.; César, G.; Raffard, G.; Gontard, N.; Gastaldi, E. How Performance and Fate of Biodegradable Mulch Films are Impacted by Field Ageing. J. Polym. Environ. 2018, 26, 2588–2600. [Google Scholar] [CrossRef]
- Freitas, A.L.P.D.L.; Tonini Filho, L.R.; Calvão, P.S.; de Souza, A.M.C. Effect of montmorillonite and chain extender on rheological, morphological and biodegradation behavior of PLA/PBAT blends. Polym. Test. 2017, 62, 189–195. [Google Scholar] [CrossRef]
- Scaffaro, R.; Maio, A.; Sutera, F.; Gulino, E.F.; Morreale, M. Degradation and recycling of films based on biodegradable polymers: A short review. Polymers 2019, 11, 651. [Google Scholar] [CrossRef] [Green Version]
- Muthuraj, R.; Misra, M.; Mohanty, A.K. Hydrolytic degradation of biodegradable polyesters under simulated environmental conditions. J. Appl. Polym. Sci. 2015, 132, 42189. [Google Scholar] [CrossRef]
- Kijchavengkul, T.; Auras, R.; Rubino, M.; Selke, S.; Ngouajio, M.; Fernandez, R.T. Biodegradation and hydrolysis rate of aliphatic aromatic polyester. Polym. Degrad. Stab. 2010, 95, 2641–2647. [Google Scholar] [CrossRef]
- Tham, W.L.; Poh, B.T.; Mohd Ishak, Z.A.; Chow, W.S. Water Absorption Kinetics and Hygrothermal Aging of Poly(lactic acid) Containing Halloysite Nanoclay and Maleated Rubber. J. Polym. Environ. 2015, 23, 242–250. [Google Scholar] [CrossRef]
- Pan, H.; Li, Z.; Yang, J.; Li, X.; Ai, X.; Hao, Y.; Zhang, H.; Dong, L. The effect of MDI on the structure and mechanical properties of poly(lactic acid) and poly(butylene adipate-co-butylene terephthalate) blends. RSC Adv. 2018, 8, 4610–4623. [Google Scholar] [CrossRef] [Green Version]
- Weng, Y.X.; Jin, Y.J.; Meng, Q.Y.; Wang, L.; Zhang, M.; Wang, Y.Z. Biodegradation behavior of poly(butylene adipate-co-terephthalate) (PBAT), poly(lactic acid) (PLA), and their blend under soil conditions. Polym. Test. 2013, 32, 918–926. [Google Scholar] [CrossRef]
- Endres, H.-J.; Siebert-Raths, A.; Endres, H.-J.; Siebert-Raths, A. Engineering Biopolymers. Eng. Biopolym. 2011, 71148, 3–15. [Google Scholar] [CrossRef]
- Itävaara, M.; Karjomaa, S.; Selin, J.F. Biodegradation of polylactide in aerobic and anaerobic thermophilic conditions. Chemosphere 2002, 46, 879–885. [Google Scholar] [CrossRef]







| Analysis | Compost |
|---|---|
| Total dry solids (%) 1 | 55 |
| Volatile solids (%) 2 | 53 |
| pH of the compost solution | 7.1 |
| Total organic carbon content (%) | 10.6 |
| Total nitrogen (%) | 0.9 |
| Carbon/nitrogen ratio | 11.8 |
| Number of Days | C–O (Wavenumber at 1710–1712 cm−1) | C–H (Wavenumber at 1452 cm−1) | Ico |
|---|---|---|---|
| PBAT–PLA | |||
| 0 | 64.89 | 11.86 | 4.41 |
| 30 | 66.14 | 12.06 | 5.48 |
| 60 | 222.96 | 12.56 | 9.88 |
| PBAT–PBS | |||
| 0 | 50.42 | 10.18 | 4.95 |
| 30 | 45.12 | 10.35 | 4.36 |
| 60 | 34.92 | 10.33 | 3.32 |
| Sample | Degradation Period (Days) | Tonset (°C) | Tminor (°C) | Tmax (°C) | Residue (%) |
|---|---|---|---|---|---|
| PLA | 0 | 321.6 | 369.4 | 0.1 | |
| PBAT | 0 | 356.4 | - | 406.4 | 0.1 |
| PBS | 0 | 358.0 | - | 399.6 | 0.1 |
| PBAT–PLA | 0 | 350.0 | - | 415.3 | 0.6 |
| 30 | 346.9 | 367.5 | 411.7 | 0.4 | |
| 60 | 171.0 | 363.5 | 402.1 | 13.1 | |
| PBAT–PBS | 0 | 356.5 | 276.2 | 409.2 | 0.2 |
| 30 | 351.1 | - | 409.6 | 2.0 | |
| 60 | 320.3 | - | 406.1 | 18.3 |
| Sample | Degradation Period (Days) | Tg (°C) | Tc (°C) | ∆H (J/g) | Tm (°C) | Xc (%) |
|---|---|---|---|---|---|---|
| PLA | 0 | 59.8 | 118.5 | 31.8 | 152.6; 160.3 | 33.94 |
| PBAT | 0 | n.d | 69.8 | 14.8 | 121.4 | 12.98 |
| PBS | 0 | n.d | 82.8 | 66.0 | 116.3 | 59.79 |
| PBAT–PLA | 0 | 59.64 | 51.98 | 15.8 | 110.4; 155.1 | 13.86 |
| 30 | 59.1 | 56.2 | 10.5 | 110.6; 155.3 | 9.25 | |
| 60 | n.d | 78.2 | 14.4 | 109.9; 143.8 | 12.63 | |
| PBAT–PBS | 0 | n.d | 90.3 | 45.9 | 105.9; 114.0 | 41.62 |
| 30 | n.d | 85.4 | 42.9 | 114.2 | 38.90 | |
| 60 | n.d | 78 | 28.5 | 112.4 | 25.85 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nomadolo, N.; Dada, O.E.; Swanepoel, A.; Mokhena, T.; Muniyasamy, S. A Comparative Study on the Aerobic Biodegradation of the Biopolymer Blends of Poly(butylene succinate), Poly(butylene adipate terephthalate) and Poly(lactic acid). Polymers 2022, 14, 1894. https://doi.org/10.3390/polym14091894
Nomadolo N, Dada OE, Swanepoel A, Mokhena T, Muniyasamy S. A Comparative Study on the Aerobic Biodegradation of the Biopolymer Blends of Poly(butylene succinate), Poly(butylene adipate terephthalate) and Poly(lactic acid). Polymers. 2022; 14(9):1894. https://doi.org/10.3390/polym14091894
Chicago/Turabian StyleNomadolo, Nomvuyo, Omotola Esther Dada, Andri Swanepoel, Teboho Mokhena, and Sudhakar Muniyasamy. 2022. "A Comparative Study on the Aerobic Biodegradation of the Biopolymer Blends of Poly(butylene succinate), Poly(butylene adipate terephthalate) and Poly(lactic acid)" Polymers 14, no. 9: 1894. https://doi.org/10.3390/polym14091894