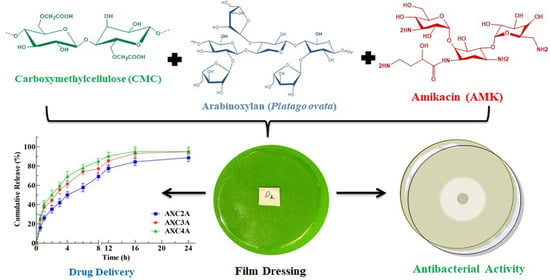
Arabinoxylan-Carboxymethylcellulose Composite Films for Antibiotic Delivery to Infected Wounds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of AMK-Loaded AX-CMC Composite Films
2.3. Physiochemical Characterization of the AX-CMC Films
2.4. Pharmaceutical Characterization of AMK-Loaded AX-CMC Composite Films
2.5. Antimicrobial Activity of AMK-Loaded AX-CMC Composite Films
2.6. Cell Viability of AMK-Loaded AX-CMC Composite Films
2.7. Statistical Analysis
3. Results and Discussion
3.1. Preparation of AMK-Loaded AX-CMC Composite Films
3.2. Physiochemical Characterization of AMK-Loaded AX-CMC Films
3.3. Pharmaceutical Characterization
3.4. Antimicrobial Activity
3.5. Cell Viability Assay of AX-CMC Composite Films
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharma, A.; Puri, V.; Kumar, P.; Singh, I. Rifampicin-loaded alginate-gelatin fibers incorporated within transdermal films as a fiber-in-film system for wound healing applications. Membranes 2021, 11, 7. [Google Scholar] [CrossRef] [PubMed]
- Michalska-Sionkowska, M.; Warżyńska, O.; Kaczmarek-Szczepańska, B.; Łukowicz, K.; Osyczka, A.M.; Walczak, M. Characterization of Collagen/Beta Glucan Hydrogels Crosslinked with Tannic Acid. Polymers 2021, 13, 3412. [Google Scholar] [CrossRef] [PubMed]
- Michalska-Sionkowska, M.; Kaczmarek, B.; Walczak, M.; Sionkowska, A. Antimicrobial activity of new materials based on the blends of collagen/chitosan/hyaluronic acid with gentamicin sulfate addition. Mater. Sci. Eng. C 2018, 86, 103–108. [Google Scholar] [CrossRef]
- Bialik-Wąs, K.; Pluta, K.; Malina, D.; Barczewski, M.; Malarz, K.; Mrozek-Wilczkiewicz, A. Advanced SA/PVA-based hydrogel matrices with prolonged release of Aloe vera as promising wound dressings. Mater. Sci. Eng. C 2021, 120, 111667. [Google Scholar] [CrossRef] [PubMed]
- Kanikireddy, V.; Varaprasad, K.; Jayaramudu, T.; Karthikeyan, C.; Sadiku, R. Carboxymethyl cellulose-based materials for infection control and wound healing: A review. Int. J. Biol. Macromol. 2020, 164, 963–975. [Google Scholar] [CrossRef] [PubMed]
- Jantrawut, P.; Bunrueangtha, J.; Suerthong, J.; Kantrong, N. Fabrication and characterization of low methoxyl pectin/gelatin/carboxymethyl cellulose absorbent hydrogel film for wound dressing applications. Materials 2019, 12, 1628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurczewska, J.; Sawicka, P.; Ratajczak, M.; Gajęcka, M.; Schroeder, G. Will the use of double barrier result in sustained release of vancomycin? Optimization of parameters for preparation of a new antibacterial alginate-based modern dressing. Int. J. Pharm. 2015, 496, 526–533. [Google Scholar] [CrossRef]
- Maver, T.; Hribernik, S.; Mohan, T.; Smrke, D.M.; Maver, U.; Stana-Kleinschek, K. Functional wound dressing materials with highly tunable drug release properties. RSC Adv. 2015, 5, 77873–77884. [Google Scholar] [CrossRef] [Green Version]
- Basu, P.; Narendrakumar, U.; Arunachalam, R.; Devi, S.; Manjubala, I. Characterization and evaluation of carboxymethyl cellulose-based films for healing of full-thickness wounds in normal and diabetic rats. ACS Omega 2018, 3, 12622–12632. [Google Scholar] [CrossRef] [Green Version]
- Kalaycıoğlu, Z.; Kahya, N.; Adımcılar, V.; Kaygusuz, H.; Torlak, E.; Akın-Evingür, G.; Erim, F.B. Antibacterial nano cerium oxide/chitosan/cellulose acetate composite films as potential wound dressing. Eur. Polym. J. 2020, 133, 109777. [Google Scholar] [CrossRef]
- Alavi, T.; Rezvanian, M.; Ahmad, N.; Mohamad, N.; Ng, S.-F. Pluronic-F127 composite film loaded with erythromycin for wound application: Formulation, physicomechanical and in vitro evaluations. Drug Deliv. Transl. Res. 2019, 9, 508–519. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Ahmad, M.M.; Alruwaili, N.K.; Alrowaili, Z.A.; Alomar, F.A.; Akhtar, S.; Alsaidan, O.A.; Alhakamy, N.A.; Zafar, A.; Elmowafy, M.; et al. Antibiotic-Loaded Psyllium Husk Hemicellulose and Gelatin-Based Polymeric Films for Wound Dressing Application. Pharmaceutics 2021, 13, 236. [Google Scholar] [CrossRef]
- Ahmad, N.; Tayyeb, D.; Ali, I.; Alruwaili, N.K.; Ahmad, W.; ur Rehman, A.; Khan, A.H.; Iqbal, M.S. Development and Characterization of Hemicellulose-Based Films for Antibacterial Wound-Dressing Application. Polymers 2020, 12, 548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simões, D.; Miguel, S.P.; Ribeiro, M.P.; Coutinho, P.; Mendonça, A.G.; Correia, I.J. Recent advances on antimicrobial wound dressing: A review. Eur. J. Pharm. Biopharm. 2018, 127, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Rezvanian, M.; Ahmad, N.; Mohd Amin, M.C.I.; Ng, S.-F. Optimization, characterization, and in vitro assessment of alginate-pectin ionic cross-linked hydrogel film for wound dressing applications. Int. J. Biol. Macromol. 2017, 97, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Hubner, P.; Donati, N.; Quines, L.K.d.M.; Tessaro, I.C.; Marcilio, N.R. Gelatin-based films containing clinoptilolite-Ag for application as wound dressing. Mater. Sci. Eng. C 2020, 107, 110215. [Google Scholar] [CrossRef] [PubMed]
- Alzarea, A.I.; Alruwaili, N.K.; Ahmad, M.M.; Munir, M.U.; Butt, A.M.; Alrowaili, Z.A.; Shahari, M.S.B.; Almalki, Z.S.; Alqahtani, S.S.; Dolzhenko, A.V.; et al. Development and Characterization of Gentamicin-Loaded Arabinoxylan-Sodium Alginate Films as Antibacterial Wound Dressing. Int. J. Mol. Sci. 2022, 23, 2899. [Google Scholar] [CrossRef] [PubMed]
- Javanbakht, S.; Shaabani, A. Carboxymethyl cellulose-based oral delivery systems. Int. J. Biol. Macromol. 2019, 133, 21–29. [Google Scholar] [CrossRef]
- Koneru, A.; Dharmalingam, K.; Anandalakshmi, R. Cellulose based nanocomposite hydrogel films consisting of sodium carboxymethylcellulose–grapefruit seed extract nanoparticles for potential wound healing applications. Int. J. Biol. Macromol. 2020, 148, 833–842. [Google Scholar] [CrossRef]
- Abdollahi, M.; Damirchi, S.; Shafafi, M.; Rezaei, M.; Ariaii, P. Carboxymethyl cellulose-agar biocomposite film activated with summer savory essential oil as an antimicrobial agent. Int. J. Biol. Macromol. 2019, 126, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Rezvanian, M.; Amin, M.C.I.M.; Ng, S.-F. Development and physicochemical characterization of alginate composite film loaded with simvastatin as a potential wound dressing. Carbohydr. Polym. 2016, 137, 295–304. [Google Scholar] [CrossRef]
- Savencu, I.; Iurian, S.; Porfire, A.; Bogdan, C.; Tomuță, I. Review of advances in polymeric wound dressing films. React. Funct. Polym. 2021, 168, 105059. [Google Scholar] [CrossRef]
- Fernandes, C.; Acharya, P.C.; Bhatt, S. Preparation of lauroyl grafted alginate-psyllium husk gel composite film with enhanced physicochemical, mechanical and antimicrobial properties. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maxwell, A.; Ghate, V.; Aranjani, J.; Lewis, S. Breaking the barriers for the delivery of amikacin: Challenges, strategies, and opportunities. Life Sci. 2021, 284, 119883. [Google Scholar] [CrossRef] [PubMed]
- Noel, S.P.; Courtney, H.S.; Bumgardner, J.D.; Haggard, W.O. Chitosan sponges to locally deliver amikacin and vancomycin: A pilot in vitro evaluation. Clin. Orthop. Relat. Res. 2010, 468, 2074–2080. [Google Scholar] [CrossRef] [Green Version]
- Boles, L.R.; Awais, R.; Beenken, K.E.; Smeltzer, M.S.; Haggard, W.O.; Jessica, A.J. Local delivery of amikacin and vancomycin from chitosan sponges prevent polymicrobial implant-associated biofilm. Mil. Med. 2018, 183, 459–465. [Google Scholar] [CrossRef] [Green Version]
- Hu, C.; Zhang, F.; Long, L.; Kong, Q.; Luo, R.; Wang, Y. Dual-responsive injectable hydrogels encapsulating drug-loaded micelles for on-demand antimicrobial activity and accelerated wound healing. J. Control. Release 2020, 324, 204–217. [Google Scholar] [CrossRef]
- Abbasi, A.R.; Sohail, M.; Minhas, M.U.; Khaliq, T.; Kousar, M.; Khan, S.; Hussain, Z.; Munir, A. Bioinspired sodium alginate based thermosensitive hydrogel membranes for accelerated wound healing. Int. J. Biol. Macromol. 2020, 155, 751–765. [Google Scholar] [CrossRef]
- Saghir, S.; Iqbal, M.S.; Koschella, A.; Heinze, T. Ethylation of arabinoxylan from Ispaghula (Plantago ovata) seed husk. Carbohydr. Polym. 2009, 77, 125–130. [Google Scholar] [CrossRef]
- ASTM-D882-18; Standard Test Method for Tensile Properties of Thin Plastic Sheeting. ASTM International: West Conshohocken, PA, USA, 2018.
- Surya, T.G.; Gurupadayya, B.; Venkata, S.K. Spectrophotometric method for the determination of amikacin in pure and pharmaceutical dosage form. Int. J. Curr. Pharm. Res. 2018, 10, 38–42. [Google Scholar]
- Riss, T.L.; Moravec, R.A.; Niles, A.L.; Duellman, S.; Benink, H.A.; Worzella, T.J.; Minor, L. Cell viability assays. In Assay Guidance Manual [Internet]; Eli Lilly & Company and the National Center for Advancing Translational Sciences: Bethesda, MA, USA, 2016. [Google Scholar]
- Trevisol, T.; Fritz, A.; de Souza, S.; Bierhalz, A.; Valle, J. Alginate and carboxymethyl cellulose in monolayer and bilayer films as wound dressings: Effect of the polymer ratio. J. Appl. Polym. Sci. 2019, 136, 46941. [Google Scholar] [CrossRef]
- Bierhalz, A.C.; Moraes, Â.M. Tuning the properties of alginate—Chitosan membranes by varying the viscosity and the proportions of polymers. J. Appl. Polym. Sci. 2016, 133, 44216. [Google Scholar] [CrossRef]
- Donnadio, A.; Ambrogi, V.; Pietrella, D.; Pica, M.; Sorrentino, G.; Casciola, M. Carboxymethylcellulose films containing chlorhexidine–zirconium phosphate nanoparticles: Antibiofilm activity and cytotoxicity. RSC Adv. 2016, 6, 46249–46257. [Google Scholar] [CrossRef]
- Thomas, D.; Nath, M.S.; Mathew, N.; Reshmy, R.; Philip, E.; Latha, M. Alginate film modified with aloevera gel and cellulose nanocrystals for wound dressing application: Preparation, characterization and in vitro evaluation. J. Drug Deliv. Sci. Technol. 2020, 59, 101894. [Google Scholar] [CrossRef]
- Antonova, N.; Babichev, A.; Berezovsky, V. Study of the morphology and structure of porous composites obtained from Na–CMC suspensions with aluminum micro-particles and boehmite nanoparticles. J. Surf. Investig. X-Ray Synchrotron Neutron Tech. 2017, 11, 955–959. [Google Scholar] [CrossRef]
- Guidi, A.C.; de Paula, M.N.; Mosela, M.; Delanora, L.A.; Soares, G.C.A.; de Morais, G.R.; de Medeiros, D.C.; Junior, A.G.d.O.; Novello, C.R.; Baesso, M.L. Stem bark extract of Poincianella pluviosa incorporated in polymer film: Evaluation of wound healing and anti-staphylococcal activities. Injury 2020, 51, 840–849. [Google Scholar] [CrossRef] [PubMed]
- Taheri, P.; Jahanmardi, R.; Koosha, M.; Abdi, S. Physical, mechanical and wound healing properties of chitosan/gelatin blend films containing tannic acid and/or bacterial nanocellulose. Int. J. Biol. Macromol. 2020, 154, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Sakthiguru, N.; Sithique, M.A. Fabrication of bioinspired chitosan/gelatin/allantoin biocomposite film for wound dressing application. Int. J. Biol. Macromol. 2020, 152, 873–883. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Eid, M.; Xiong, W.; Ren, C.; Ai, T.; Deng, Z.; Li, J.; Li, B. Comparative study between cold and hot water extracted polysaccharides from Plantago ovata seed husk by using rheological methods. Food Hydrocoll. 2020, 101, 105465. [Google Scholar] [CrossRef]
- Saadiah, M.A.; Zhang, D.; Nagao, Y.; Muzakir, S.K.; Samsudin, A.S. Reducing crystallinity on thin film based CMC/PVA hybrid polymer for application as a host in polymer electrolytes. J. Non-Cryst. Solids 2019, 511, 201–211. [Google Scholar] [CrossRef]
- Kaur, A.; Kumar, R. Enhanced bactericidal efficacy of polymer stabilized silver nanoparticles in conjugation with different classes of antibiotics. RSC Adv. 2019, 9, 1095–1105. [Google Scholar] [CrossRef] [Green Version]
- Salama, A. Preparation of CMC-g-P(SPMA) super adsorbent hydrogels: Exploring their capacity for MB removal from waste water. Int. J. Biol. Macromol. 2018, 106, 940–946. [Google Scholar] [CrossRef]
- Bajpai, S.K.; Chand, N.; Ahuja, S. Investigation of curcumin release from chitosan/cellulose micro crystals (CMC) antimicrobial films. Int. J. Biol. Macromol. 2015, 79, 440–448. [Google Scholar] [CrossRef]
- Sood, S.; Gupta, V.K.; Agarwal, S.; Dev, K.; Pathania, D. Controlled release of antibiotic amoxicillin drug using carboxymethyl cellulose-cl-poly(lactic acid-co-itaconic acid) hydrogel. Int. J. Biol. Macromol. 2017, 101, 612–620. [Google Scholar] [CrossRef]
- Sharma, U.K.; Verma, A.; Prajapati, S.K.; Pandey, H.; Pandey, A.C. In vitro, in vivo and pharmacokinetic assessment of amikacin sulphate laden polymeric nanoparticles meant for controlled ocular drug delivery. Appl. Nanosci. 2015, 5, 143–155. [Google Scholar] [CrossRef] [Green Version]
- Malik, N.S.; Ahmad, M.; Minhas, M.U. Cross-linked β-cyclodextrin and carboxymethyl cellulose hydrogels for controlled drug delivery of acyclovir. PLoS ONE 2017, 12, e0172727. [Google Scholar] [CrossRef] [Green Version]
- Varshosaz, J.; Ghaffari, S.; Khoshayand, M.R.; Atyabi, F.; Dehkordi, A.J.; Kobarfard, F. Optimization of freeze-drying condition of amikacin solid lipid nanoparticles using D-optimal experimental design. Pharm. Dev. Technol. 2012, 17, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, S.; Daheriya, P.; Ahuja, S.; Gupta, K. Water absorption and antimicrobial behavior of physically cross linked poly (vinyl alcohol)/carrageenan films loaded with minocycline. Des. Monomers Polym. 2016, 19, 630–642. [Google Scholar] [CrossRef] [Green Version]
- Matthews, K.H.; Stevens, H.N.E.; Auffret, A.D.; Humphrey, M.J.; Eccleston, G.M. Lyophilised wafers as a drug delivery system for wound healing containing methylcellulose as a viscosity modifier. Int. J. Pharm. 2005, 289, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Vinklárková, L.; Masteiková, R.; Vetchý, D.; Doležel, P.; Bernatonienė, J. Formulation of Novel Layered Sodium Carboxymethylcellulose Film Wound Dressings with Ibuprofen for Alleviating Wound Pain. BioMed. Res. Int. 2015, 2015, 892671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Setapa, A.; Ahmad, N.; Mahali, S.M.; Amin, M.C.I.M. Mathematical Model for Estimating Parameters of Swelling Drug Delivery Devices in a Two-Phase Release. Polymers 2020, 12, 2921. [Google Scholar] [CrossRef]
- Zhang, F.; Yang, H.; Yang, Y.; Wang, H.; Li, X.; Wu, X. Stretchable and biocompatible bovine serum albumin fibrous films supported silver for accelerated bacteria-infected wound healing. Chem. Eng. J. 2021, 417, 129145. [Google Scholar] [CrossRef]






| Film Code | Composition (% w/w) | Physical Characteristics | |||||
|---|---|---|---|---|---|---|---|
| AX | CMC | GLY | AMK | Peelability | Foldability | Transparency | |
| AXC1 | 2 | 2 | 1 | - | ✓ | ✘ | ✓ |
| AXC2 | 2 | 2 | 2 | - | ✓ | ✓ | ✓ |
| AXC3 | 2 | 1.5 | 2 | - | ✓ | ✓ | ✓ |
| AXC4 | 1.5 | 2 | 2 | - | ✓ | ✓ | ✓ |
| AXC5 | 1.5 | 1.5 | 2 | - | ✘ | ✘ | ✓ |
| AXC2A | 2 | 2 | 2 | 0.2 | ✓ | ✓ | ✓ |
| AXC3A | 2 | 1.5 | 2 | 0.2 | ✓ | ✓ | ✓ |
| AXC4A | 1.5 | 2 | 2 | 0.2 | ✓ | ✓ | ✓ |
| Film | Thickness (µm) | Water loss (%) | WVPR (g m−2 day−1) | Tensile Strength (MPa) | EB (%) |
|---|---|---|---|---|---|
| AXC2 | 147 ± 4.1 | 94.9 ± 1.8 | 1157 ± 79 | 2.98 ± 0.21 | 118 ± 5.3 |
| AXC3 | 146 ± 4.2 | 95.1 ± 1.2 | 1441± 94 # | 2.52 ± 0.14 ‡ | 97 ± 6.1 ‡ |
| AXC4 | 147 ± 5.3 | 93.7 ± 1.6 | 1424 ± 74 # | 2.93 ± 0.12 | 117 ± 5.6 |
| AXC2A | 158 ± 4.5 * | 93.7 ± 1.2 | 1142 ± 89 | 3.41 ± 0.13 | 114 ± 4.7 |
| AXC3A | 156 ± 3.7 * | 94.5 ± 1.5 | 1495 ± 71 # | 2.57 ± 0.15 ‡ | 93 ± 4.5 ‡ |
| AXC4A | 158 ± 4.5 * | 94.4 ± 1.1 | 1489 ± 91 # | 3.00 ± 0.13 | 113 ± 3.7 |
| Mathematical Model | AXC2A | AXC3A | AXC4A | |
|---|---|---|---|---|
| Zero-order | R2 | 0.8229 | 0.7641 | 0.3866 |
| Ko | 2.9836 | 2.7831 | 2.6271 | |
| First-order | R2 | 0.9611 | 0.9557 | 0.9611 |
| K1 | −0.0382 | −0.0516 | −0.0380 | |
| Higuchi | R2 | 0.9535 | 0.9182 | 0.8666 |
| KH | 18.046 | 17.143 | 16.612 | |
| Korsmeyer-Peppas | R2 | 0.9850 | 0.9853 | 0.9821 |
| KKP | 1.3861 | 1.5177 | 1.5634 | |
| n | 0.5248 | 0.4771 | 0.4642 | |
| Hixson-Crowell | R2 | 0.8229 | 0.7641 | 0.6873 |
| KHC | −0.9945 | −0.9277 | 0.8776 |
| Code | S. aureus (mm) | E. coli (mm) | P. aeruginosa (mm) |
|---|---|---|---|
| Blank Disk | 9.1 ± 3.8 | 6.8 ± 0.1 | 6.8 ± 0.1 |
| Blank Film | 7.7 ± 0.5 | 7.1 ± 0.3 | 6.99 ± 0.2 |
| AXC2A | 32.9 ± 3.2 * | 31.3 ± 3.1 * | 29.4 ± 3.4 * |
| AXC3A | 27.2 ± 4.7 * | 28.8 ± 2.8 * | 25.1 ± 2.9 * |
| AXC4A | 31.5 ± 3.9 * | 30.2 ± 4.1 * | 29.3 ± 3.7 * |
| AMK Std. | 29.9 ± 4.3 * | 30.1 ± 3.9 * | 30.2 ± 2.7 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alruwaili, N.K.; Ahmad, N.; Alzarea, A.I.; Alomar, F.A.; Alquraini, A.; Akhtar, S.; Shahari, M.S.B.; Zafar, A.; Elmowafy, M.; Elkomy, M.H.; et al. Arabinoxylan-Carboxymethylcellulose Composite Films for Antibiotic Delivery to Infected Wounds. Polymers 2022, 14, 1769. https://doi.org/10.3390/polym14091769
Alruwaili NK, Ahmad N, Alzarea AI, Alomar FA, Alquraini A, Akhtar S, Shahari MSB, Zafar A, Elmowafy M, Elkomy MH, et al. Arabinoxylan-Carboxymethylcellulose Composite Films for Antibiotic Delivery to Infected Wounds. Polymers. 2022; 14(9):1769. https://doi.org/10.3390/polym14091769
Chicago/Turabian StyleAlruwaili, Nabil K., Naveed Ahmad, Abdulaziz I. Alzarea, Fadhel A. Alomar, Ali Alquraini, Sultan Akhtar, Muhammad Syafiq Bin Shahari, Ameeduzzafar Zafar, Mohammed Elmowafy, Mohammed H. Elkomy, and et al. 2022. "Arabinoxylan-Carboxymethylcellulose Composite Films for Antibiotic Delivery to Infected Wounds" Polymers 14, no. 9: 1769. https://doi.org/10.3390/polym14091769