Preparation and Properties of Sulfonated Poly(phthalazinone ether ketone) Membranes for Electrodialysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of SPPEK
2.3. Membrane Preparation
2.4. Characterization
2.4.1. Polymer Characterization
2.4.2. Thermal Stability of Polymer
2.4.3. Ion Exchange Capacity (IEC)
2.4.4. Water Uptake and Swelling Rate
2.4.5. Mechanical Properties
2.4.6. Scanning Electron Microscopy (SEM)
2.4.7. Membrane Area Resistance
2.4.8. Transport Number
2.4.9. Electrodialysis Experiment
2.4.10. Current–Voltage Curves Measurement
2.4.11. Saline Water Treatment
3. Results and Discussion
3.1. Synthesis of SPPEK and Chemical Structure Characterization
3.2. Thermal Analysis
3.3. Morphology and Mechanical Properties of the Membranes
3.4. Electrochemical Properties of the Membranes
3.5. Limiting Current Density of the Membrane
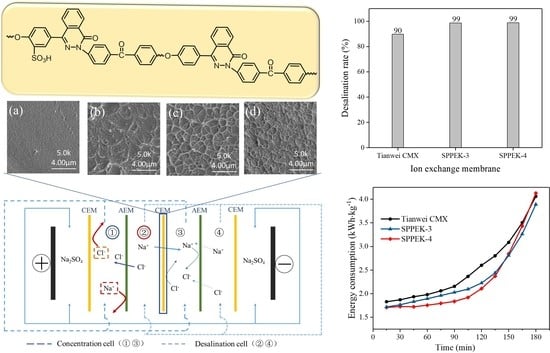
3.6. Electrodialysis Experiment
3.7. Saline Water Treatment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nagarale, R.K.; Gohil, G.S. Recent developments on ion-exchange membranes and electro-membrane processes. Adv. Colloid Interface Sci. 2006, 119, 97–130. [Google Scholar] [CrossRef]
- Wirth, M.A.; Sievers, M. Electrodialysis as a sample processing tool for bulk organic matter and target pollutant analysis of seawater. Mar. Chem. 2019, 217, 103719. [Google Scholar] [CrossRef]
- Gmar, S.; Chagnes, A. Recent advances on electrodialysis for the recovery of lithium from primary and secondary resources. Hydrometallurgy 2019, 189, 105124–105135. [Google Scholar] [CrossRef]
- Cong, M.; Jia, Y. Preparation of acid block anion exchange membrane with quaternary ammonium groups by homogeneous amination for electrodialysis-based acid enrichment. Sep. Purif. Technol. 2019, 238, 116396. [Google Scholar] [CrossRef]
- Luo, J.; Wu, C. Diffusion dialysis-concept, principle and applications. J. Membr. Sci. 2011, 366, 1–16. [Google Scholar] [CrossRef]
- Karimi, L.; Ghassemi., A. Quantitative studies of electrodialysis performance. Desalination 2018, 445, 159–169. [Google Scholar] [CrossRef]
- Ye, Z.; Ghyselbrecht, K. Fractionating magnesium ion from seawater for struvite recovery using electrodialysis with monovalent selective membranes. Chemosphere 2018, 210, 867–876. [Google Scholar] [CrossRef]
- Strathmann, H.; Grabowski, A. Ion-Exchange Membranes in the Chemical Process Industry. Ind. Eng. Chem. Res. 2013, 52, 10364–10379. [Google Scholar] [CrossRef]
- Liu, Q.; Li, X. Novel sulfonated N-heterocyclic poly(aryl ether ketone ketone)s with pendant phenyl groups for proton exchange membrane performing enhanced oxidative stability and excellent fuel cell properties. J. Membr. Sci. 2022, 641, 119926. [Google Scholar] [CrossRef]
- Liu, C.; Li, X. Synthesis and characterization of sulfonated polybenzimidazoles containing 4-phenyl phthalazinone groups for proton exchange membrane. Solid State Ion. 2014, 261, 67–73. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, S. Quaternized poly(phthalazinone ether ketone ketone) anion exchange membrane with low permeability of vanadium ions for vanadium redox flow battery application. J. Power Source. 2012, 217, 296–302. [Google Scholar] [CrossRef]
- Zheng, P.; Liu, Q. Effect of Crosslinking Degree on Sulfonated Poly(aryl ether nitrile)s As Candidates for Proton Exchange Membranes. Polymers 2019, 11, 964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.; Zhou, J. Synthesis and properties of sulfonated poly(ether ketone)s containing 3,5-dimethyl phthalazinone moieties as proton exchange membrane materials. Chin. J. Polym. Sci. 2012, 30, 511–519. [Google Scholar] [CrossRef]
- Koromilas, N.D.; Anastasopoul, C. Preparation of Porous Polymeric Membranes Based on a Pyridine Containing Aromatic Polyether Sulfone. Polymers 2019, 11, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, N.; Liu, Y. Polymer inclusion membrane (PIM) containing ionic liquid as a proton blocker to improve waste acid recovery efficiency in electrodialysis process. J. Membr. Sci. 2019, 581, 18–27. [Google Scholar] [CrossRef]
- Sun, X.; Lu, H. Recovery of citric acid from fermented liquid by bipolar membrane electrodialysis. J. Clean. Prod. 2017, 143, 250–256. [Google Scholar] [CrossRef]
- Strathmann, H. Electrodialysis, a mature technology with a multitude of new applications. Desalination 2010, 264, 268–288. [Google Scholar] [CrossRef]
- Lee, H.; Song, J. Comparison of electrodialysis reversal (EDR) and electrodeionization reversal (EDIR) for water softening. Desalination 2013, 314, 43–49. [Google Scholar] [CrossRef]
- Moser, P.B.; Ricci, B.C. Removal of organic matter of electrodialysis reversal brine from a petroleum refinery wastewater reclamation plant by UV and UV/H2O2 process. J. Environ. Sci. Health Part A-Toxic/Hazard. Subst. Environ. Eng. 2018, 53, 430–435. [Google Scholar]
- Yao, Z.; Cui, M. Silane Cross-Linked Sulfonted Poly(Ether Ketone/Ether Benzimidazole)s for Fuel Cell Applications. Polymers 2017, 9, 631. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.; Wu, H. Anion Conductive Triblock Copolymer Membranes with Flexible Multication Side Chain. ACS Appl. Mater. Interfaces 2018, 10, 18327–18337. [Google Scholar] [CrossRef]
- Rodgers, M.P.; Bonville, L.J. Fuel Cell Perfluorinated Sulfonic Acid Membrane Degradation Correlating Accelerated Stress Testing and Lifetime. Chem. Rev. 2012, 112, 6075–6103. [Google Scholar] [CrossRef]
- Garcia, N.D.; Maria, B.V. A comparative study of the electro-osmotic behavior of cation and anion exchange membranes in alcohol-water media. Electrochim. Acta 2015, 154, 166–176. [Google Scholar] [CrossRef]
- Martos, A.M.; Sanchez, J.Y. Electrochemical and structural characterization of sulfonated polysulfone. Polym. Test. 2015, 45, 185–193. [Google Scholar] [CrossRef] [Green Version]
- Kenneth, A.M.; Robert, B.M. State of Understanding of Nafion. Chem. Rev. 2004, 104, 4535–4580. [Google Scholar]
- Su, L.; Zhang, D. Orientated graphene oxide/Nafion ultra-thin layer coated composite membranes for vanadium redox flow battery. Int. J. Hydrogen Energy 2017, 42, 21806–21816. [Google Scholar] [CrossRef]
- Feng, S.; Shen, K. Concentrated sulfonated poly (ether sulfone)s as proton exchange membranes. J. Power Sources 2013, 224, 42–49. [Google Scholar] [CrossRef]
- Zhao, J.; Sun, L. Modification of cation exchange membranes with conductive polyaniline for electrodialysis applications. J. Membr. Sci. 2019, 582, 211–223. [Google Scholar] [CrossRef]
- Simari, C.; Prejanò, M. Exploring the Structure–Performance Relationship of Sulfonated Polysulfone Proton Exchange Membrane by a Combined Computational and Experimental Approach. Polymers 2021, 13, 959. [Google Scholar] [CrossRef]
- Luo, Q.; Zhang, H. Preparation and characterization of Nafion/SPEEK layered composite membrane and its application in vanadium redox flow battery. J. Membr. Sci. 2008, 325, 553–558. [Google Scholar] [CrossRef]
- Wang, Y.; Peng, J. PVDF based ion exchange membrane prepared by radiation grafting of ethyl styrenesulfonate and sequent hydrolysis. Radiat. Phys. Chem. 2017, 130, 252–258. [Google Scholar] [CrossRef]
- Zhou, M.; Chen, X. A novel UV-crosslinked sulphonated polysulfone cation exchange membrane with improved dimensional stability for electrodialysis. Desalination 2017, 415, 29–39. [Google Scholar] [CrossRef]
- Farrokhzad, H.; Kikhavani, T. Novel composite cation exchange films based on sulfonated PVDF for electromembrane separations. J. Membr. Sci. 2015, 474, 167–174. [Google Scholar] [CrossRef]
- Geetanjali, S.; Vinod, K.S. Sulfonated poly(ether ether ketone)/imidized graphene oxide composite cation exchange membrane with improved conductivity and stability for electrodialytic water desalination. Desalination 2018, 451, 200–208. [Google Scholar]
- Gao, Y.; Robertson, G.P. Sulfonation of poly(phthalazinones) with fuming sulfuric acid mixtures for proton exchange membrane materials. J. Membr. Sci. 2003, 227, 39–50. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Zhang, B. Anion exchange membranes from brominated poly(aryl ether ketone) containing 3,5-dimethyl phthalazinone moieties for vanadium redox flow batteries. J. Mater. Chem. A 2014, 2, 3083–3091. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, B. Modified sulfonated poly(phthalazinone ether ketone) membranes with inorganic particles for potential applications in PEMFCs. J. Appl. Polym. Sci. 2006, 102, 3972–3977. [Google Scholar]
- Chen, Y.; Zhang, S. Sulfonated component-incorporated quaternized poly(phthalazinone ether ketone) membranes with improved ion selectivity, stability and water transport resistance in a vanadium redox flow battery. RSC Adv. 2019, 9, 26097–26108. [Google Scholar] [CrossRef] [Green Version]
- Bao, F.; Zhang, F. Study of the effects of the structure of phthalazinone’s side-group on the properties of the poly(phthalazinone ether ketone)s resins. Polymers 2019, 11, 803. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Zhu, B. Composite membranes of sulfonated poly(phthalazinone ether ketone) doped with 12-phosphotungstic acid (H3PW12O40) for proton exchange membranes. Solid State Ion. 2006, 177, 1123–1128. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, S. Low vanadium ion permeabilities of sulfonated poly(phthalazinone ether ketone)s provide high efficiency and stability for vanadium redox flow batteries. J. Power Sources 2017, 355, 23–30. [Google Scholar] [CrossRef]
- Hao, L.; Wang, C. A facile approach to fabricate composite anion exchange membranes with enhanced ionic conductivity and dimensional stability for electrodialysis. Sep. Purif. Technol. 2019, 227, 115725. [Google Scholar] [CrossRef]
- Liu, Y.; Pan, Q. In-situ crosslinking of anion exchange membrane bearing unsaturated moieties for electrodialysis. Sep. Purif. Technol. 2015, 156, 226–233. [Google Scholar] [CrossRef]
- Pan, Q.; Hossain, M.M. One-pot solvent-free synthesis of cross-linked anion exchange membranes for electrodialysis. J. Membr. Sci. 2016, 515, 115–124. [Google Scholar] [CrossRef]
- Zhang, W.; Ma, J. Comparisons on test methods of diffusion boundary layer thickness. Membr. Sci. Technol. 2016, 37, 12–18. [Google Scholar]
- Guan, S.; Zhang, S. Preparation and properties of novel sulfonated copoly (phthalazinone biphenyl ether sulfone) composite nanofiltration membrane. Desalination 2013, 318, 56–63. [Google Scholar] [CrossRef]
- Jiang, C.; Wang, Q. Water electro-transport with hydrated cations in electrodialysis. Desalination 2015, 365, 204–212. [Google Scholar] [CrossRef]












| Sulfonated Polymer | PPEK (g) | Chlorosulfonic Acid (mL) | Reaction Time (h) | IEC a (mmol·g−1) | IEC b (mmol·g−1) |
|---|---|---|---|---|---|
| SPPEK-1 | 5 | 10 | 2 | 0.77 | 0.76 |
| SPPEK-2 | 5 | 10 | 3 | 1.02 | 1.04 |
| SPPEK-3 | 5 | 10 | 5 | 1.45 | 1.40 |
| SPPEK-4 | 5 | 10 | 7 | 1.82 | 1.88 |
| Membranes | Tensile Strength (MPa) | Elongation at Break (%) |
|---|---|---|
| SPPEK-1 | 84.9 | 18.2 |
| SPPEK-2 | 80.8 | 30.0 |
| SPPEK-3 | 78.7 | 52.6 |
| SPPEK-4 | 68.5 | 63.6 |
| Membranes | IEC (mmol·g−1) | Area Resistance (Ω·cm2) | Transport Number | Water Uptake (%) | Swelling Rate (%) |
|---|---|---|---|---|---|
| SPPEK-1 | 0.77 | 51.29 ± 0.27 | 0.90 | 4.7 ± 0.8 | 2.9 ± 0.1 |
| SPPEK-2 | 1.02 | 15.41 ± 0.23 | 0.93 | 9.0 ± 0.9 | 4.1 ± 0.3 |
| SPPEK-3 | 1.45 | 1.13 ± 0.18 | 0.97 | 23.0 ± 0.8 | 7.3 ± 0.8 |
| SPPEK-4 | 1.82 | 0.62 ± 0.10 | 0.97 | 40.2 ± 1.3 | 10.4 ± 1.3 |
| CMX | 1.08 | 2.35 ± 0.13 | 0.93 | 18.4 ± 2.3 | 6.4 ± 0.6 |
| Membranes | LCD (mA·cm2) | Rohm a (Ω·cm2) |
|---|---|---|
| SPPEK-1 | 17.0 | 6.75 |
| SPPEK-2 | 23.2 | 3.03 |
| SPPEK-3 | 34.3 | 1.35 |
| SPPEK-4 | 39.8 | 1.23 |
| CMX | 28.3 | 2.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, C.; Liu, Q.; Zhang, S.; Wang, Z.; Chen, Y.; Jian, X. Preparation and Properties of Sulfonated Poly(phthalazinone ether ketone) Membranes for Electrodialysis. Polymers 2022, 14, 1723. https://doi.org/10.3390/polym14091723
Deng C, Liu Q, Zhang S, Wang Z, Chen Y, Jian X. Preparation and Properties of Sulfonated Poly(phthalazinone ether ketone) Membranes for Electrodialysis. Polymers. 2022; 14(9):1723. https://doi.org/10.3390/polym14091723
Chicago/Turabian StyleDeng, Cong, Qian Liu, Shouhai Zhang, Zhaoqi Wang, Yuning Chen, and Xigao Jian. 2022. "Preparation and Properties of Sulfonated Poly(phthalazinone ether ketone) Membranes for Electrodialysis" Polymers 14, no. 9: 1723. https://doi.org/10.3390/polym14091723