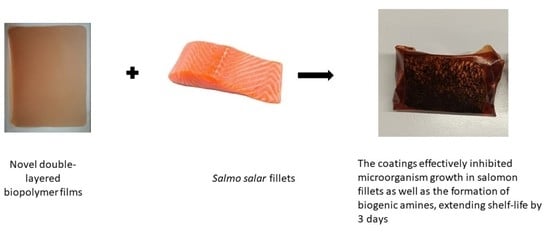
Attempt to Extend the Shelf-Life of Fish Products by Means of Innovative Double-Layer Active Biodegradable Films
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Film Preparation
2.2.2. Biodegradation Assessment of Films
Film and Compost Chemical Composition
Solubility of the Tested Films
Degree of Biodegradation
Respiratory Activity of the Material during Stimulated Biological Changes (Composting)
Ecotoxicity Testing
2.2.3. Fish Preservation Testing
Preparation of the Sample
Analyzing Microbiological Properties
Analysis of Biogenic Amines
Oxidation Rate of Fish Lipids
2.2.4. Statistical Analysis
3. Results and Discussion
3.1. Results of Biodegradation
3.2. Fish Preservation Testing
3.2.1. Microbiological Analysis
3.2.2. Content of Biogenic Amines in Fish Samples
3.2.3. Lipid Oxidation of Salmon Fillets
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Umaraw, P.; Munekata, P.E.; Verma, A.K.; Barba, F.J.; Singh, V.; Kumar, P.; Lorenzo, J.M. Edible films/coating with tailored properties for active packaging of meat, fish and derived products. Trends Food Sci. Technol. 2020, 98, 10–24. [Google Scholar] [CrossRef]
- Asioli, D.; Aschemann-Witzel, J.; Caputo, V.; Vecchio, R.; Annunziata, A.; Næs, T.; Varela, P. Making sense of the “clean label” trends: A review of consumer food choice behavior and discussion of industry implications. Food Res. Int. 2017, 99, 58–71. [Google Scholar] [CrossRef] [PubMed]
- Wong, L.-W.; Hou, C.-Y.; Hsieh, C.-C.; Chang, C.-K.; Wu, Y.-S.; Hsieh, C.-W. Preparation of antimicrobial active packaging film by capacitively coupled plasma treatment. LWT 2020, 117, 108612. [Google Scholar] [CrossRef]
- Jamróz, E.; Juszczak, L.; Kucharek, M. Investigation of the physical properties, antioxidant and antimicrobial activity of ternary potato starch-furcellaran-gelatin films incorporated with lavender essential oil. Int. J. Biol. Macromol. 2018, 114, 1094–1101. [Google Scholar] [CrossRef] [PubMed]
- Sabbagh, F.; Muhamad, I.I.; Pa’e, N.; Hashim, Z. Strategies in improving properties of cellulose-based hydrogels for smart applications. In Cellulose-Based Superabsorbent Hydrogels; Springer: Berlin/Heidelberg, Germany, 2019; pp. 887–908. [Google Scholar]
- Nastasi, J.R.; Kontogiorgos, V.; Daygon, V.D.; Fitzgerald, M.A. Pectin-based films and coatings with plant extracts as natural preservatives: A systematic review. Trends Food Sci. Technol. 2022, 120, 193–211. [Google Scholar] [CrossRef]
- Ștefănescu, B.-E.; Călinoiu, L.F.; Ranga, F.; Fetea, F.; Mocan, A.; Vodnar, D.C.; Crișan, G. Chemical composition and biological activities of the nord-west romanian wild bilberry (Vaccinium myrtillus L.) and lingonberry (Vaccinium vitis-idaea L.) leaves. Antioxidants 2020, 9, 495. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Munekata, P.E.; Gómez, B.; Barba, F.J.; Mora, L.; Pérez-Santaescolástica, C.; Toldrá, F. Bioactive peptides as natural antioxidants in food products–A review. Trends Food Sci. Technol. 2018, 79, 136–147. [Google Scholar] [CrossRef]
- Tkaczewska, J.; Jamróz, E.; Kulawik, P.; Morawska, M.; Szczurowska, K. Evaluation of the potential use of carp (Cyprinus carpio) skin gelatine hydrolysate as an antioxidant component. Food Funct. 2019, 10, 1038–1048. [Google Scholar] [CrossRef]
- Tkaczewska, J.; Bukowski, M.; Mak, P. Identification of Antioxidant Peptides in Enzymatic Hydrolysates of Carp (Cyprinus Carpio) Skin Gelatin. Molecules 2019, 24, 97. [Google Scholar] [CrossRef] [Green Version]
- Tkaczewska, J.; Kulawik, P.; Jamróz, E.; Guzik, P.; Zając, M.; Szymkowiak, A.; Turek, K. One-and double-layered furcellaran/carp skin gelatin hydrolysate film system with antioxidant peptide as an innovative packaging for perishable foods products. Food Chem. 2021, 351, 129347. [Google Scholar] [CrossRef]
- Adande, R.; Liady, M.N.D.; Gildas, D.; Césaire, A.M.; Fiogbe, E.D. A review of captures and treatments of sea food, post mortem biochemical degradations of macro-molecules and impacts of certain factors on the quality of the fish. Int. J. Fish. Aquat. Stud. 2020, 8, 351–359. [Google Scholar]
- Stejskal, N.; Miranda, J.M.; Martucci, J.F.; Ruseckaite, R.A.; Barros-Velázquez, J.; Aubourg, S.P. Quality enhancement of refrigerated hake muscle by active packaging with a protein concentrate from Spirulina platensis. Food Bioprocess Technol. 2020, 13, 1110–1118. [Google Scholar] [CrossRef]
- Jamróz, E.; Tkaczewska, J.; Juszczak, L.; Zimowska, M.; Kawecka, A.; Krzyściak, P.; Skóra, M. The influence of lingonberry extract on the properties of novel, double-layered biopolymer films based on furcellaran, CMC and a gelatin hydrolysate. Food Hydrocoll. 2021, 124, 107334. [Google Scholar] [CrossRef]
- Jamróz, E.; Tkaczewska, J.; Kopeć, M.; Cholewa-Wójcik, A. Shelf-Life Extension of Salmon Using Active Total Biodegradable Packaging with Tea Ground Waste and Furcellaran-CMC Double-Layered Films. Food Chem. 2022, 383, 132425. [Google Scholar] [CrossRef] [PubMed]
- Tkaczewska, J.; Morawska, M.; Kulawik, P.; Zając, M. Characterization of carp (Cyprinus carpio) skin gelatin extracted using different pretreatments method. Food Hydrocoll. 2018, 81, 169–179. [Google Scholar] [CrossRef]
- Gan, P.G.; Sam, S.T.; Abdullah, M.; Omar, M.F.; Tan, W.K. Water resistance and biodegradation properties of conventionally-heated and microwave-cured cross-linked cellulose nanocrystal/chitosan composite films. Polym. Degrad. Stab. 2021, 188, 109563. [Google Scholar] [CrossRef]
- Wongphan, P.; Panrong, T.; Harnkarnsujarit, N. Effect of different modified starches on physical, morphological, thermomechanical, barrier and biodegradation properties of cassava starch and polybutylene adipate terephthalate blend film. Food Packag. Shelf Life 2022, 32, 100844. [Google Scholar] [CrossRef]
- Rasool, A.; Ata, S.; Islam, A.; Rizwan, M.; Azeem, M.K.; Mehmood, A.; Khan, R.U.; Mahmood, H.A. Kinetics and controlled release of lidocaine from novel carrageenan and alginate-based blend hydrogels. Int. J. Biol. Macromol. 2020, 147, 67–78. [Google Scholar] [CrossRef]
- Beghetto, V.; Gatto, V.; Conca, S.; Bardella, N.; Buranello, C.; Gasparetto, G.; Sole, R. Development of 4-(4, 6-dimethoxy-1, 3, 5-triazin-2-yl)-4-methyl-morpholinium chloride cross-linked carboxymethyl cellulose films. Carbohydr. Polym. 2020, 249, 116810. [Google Scholar] [CrossRef]
- Harnkarnsujarit, N.; Li, Y. Structure–property modification of microcrystalline cellulose film using agar and propylene glycol alginate. J. Appl. Polym. Sci. 2017, 134, 45533. [Google Scholar] [CrossRef]
- Wongphan, P.; Khowthong, M.; Supatrawiporn, T.; Harnkarnsujarit, N. Novel edible starch films incorporating papain for meat tenderization. Food Packag. Shelf Life 2022, 31, 100787. [Google Scholar] [CrossRef]
- Nohynek, L.J.; Alakomi, H.-L.; Kähkönen, M.P.; Heinonen, M.; Helander, I.M.; Oksman-Caldentey, K.-M.; Puupponen-Pimiä, R.H. Berry phenolics: Antimicrobial properties and mechanisms of action against severe human pathogens. Nutr. Cancer 2006, 54, 18–32. [Google Scholar] [CrossRef]
- Abdelhedi, O.; Jridi, M.; Nasri, R.; Mora, L.; Toldrá, F.; Nasri, M. Rheological and structural properties of Hemiramphus far skin gelatin: Potential use as an active fish coating agent. Food Hydrocoll. 2019, 87, 331–341. [Google Scholar] [CrossRef]
- Guillén, G.; López Caballero, M.; Alemán, A.; López de Lacey, A.; Giménez, B.; Montero García, P. Antioxidant and antimicrobial peptide fractions from squid and tuna skin gelatin. In Sea By-Products as Real Material: New Ways of Application; Bihan, E.L., Ed.; Transworld Research Network: Kerala, India, 2010; pp. 89–115. [Google Scholar]
- Jridi, M.; Mora, L.; Souissi, N.; Aristoy, M.-C.; Nasri, M.; Toldrá, F. Effects of active gelatin coated with henna (L. inermis) extract on beef meat quality during chilled storage. Food Control 2018, 84, 238–245. [Google Scholar] [CrossRef]
- Pane, K.; Durante, L.; Crescenzi, O.; Cafaro, V.; Pizzo, E.; Varcamonti, M.; Zanfardino, A.; Izzo, V.; di Donato, A.; Notomista, E. Antimicrobial potency of cationic antimicrobial peptides can be predicted from their amino acid composition: Application to the detection of “cryptic” antimicrobial peptides. J. Theor. Biol. 2017, 419, 254–265. [Google Scholar] [CrossRef]
- Rai, M.; Pandit, R.; Gaikwad, S.; Kövics, G. Antimicrobial peptides as natural bio-preservative to enhance the shelf-life of food. J. Food Sci. Technol. 2016, 53, 3381–3394. [Google Scholar] [CrossRef] [Green Version]
- Verma, A.K.; Chatli, M.K.; Mehta, N.; Kumar, P. Assessment of physico-chemical, antioxidant and antimicrobial activity of porcine blood protein hydrolysate in pork emulsion stored under aerobic packaging condition at 4±1 C. LWT-Food Sci. Technol. 2018, 88, 71–79. [Google Scholar] [CrossRef]
- Özvural, E.B.; Huang, Q.; Chikindas, M.L. The comparison of quality and microbiological characteristic of hamburger patties enriched with green tea extract using three techniques: Direct addition, edible coating and encapsulation. LWT-Food Sci. Technol. 2016, 68, 385–390. [Google Scholar] [CrossRef]
- Klinmalai, P.; Srisa, A.; Laorenza, Y.; Katekhong, W.; Harnkarnsujarit, N. Antifungal and plasticization effects of carvacrol in biodegradable poly (lactic acid) and poly (butylene adipate terephthalate) blend films for bakery packaging. LWT-Food Sci. Technol. 2021, 152, 112356. [Google Scholar] [CrossRef]
- Tumbarski, Y.D.; Todorova, M.M.; Topuzova, M.G.; Georgieva, P.I.; Ganeva, Z.A.; Mihov, R.B.; Yanakieva, V.B. Antifungal activity of carboxymethyl cellulose edible films enriched with propolis extracts and their role in improvement of the storage life of Kashkaval cheese. Curr. Res. Nutr. Food Sci. 2021, 9, 487. [Google Scholar] [CrossRef]
- Ojagh, S.M.; Rezaei, M.; Razavi, S.H.; Hosseini, S.M.H. Effect of chitosan coatings enriched with cinnamon oil on the quality of refrigerated rainbow trout. Food Chem. 2010, 120, 193–198. [Google Scholar] [CrossRef]
- HPA, H. Guidelines for Assessing the Microbiological Safety of Ready-to-Eat Foods Placed on the Market; HPA: London, UK, 2009. [Google Scholar]
- Zhao, X.; Chen, L.; Wongmaneepratip, W.; He, Y.; Zhao, L.; Yang, H. Effect of vacuum impregnated fish gelatin and grape seed extract on moisture state, microbiota composition, and quality of chilled seabass fillets. Food Chem. 2021, 354, 129581. [Google Scholar] [CrossRef] [PubMed]
- Chatkitanan, T.; Harnkarnsujarit, N. Effects of nitrite incorporated active films on quality of pork. Meat Sci. 2021, 172, 108367. [Google Scholar] [CrossRef] [PubMed]
- Demircan, B.; Özdestan Ocak, Ö. The effects of ethyl lauroyl arginate and lemon essential oil added edible chitosan film coating on biogenic amines formation during storage in mackerel fillets. J. Food Process. Preserv. 2021, 45, e15454. [Google Scholar] [CrossRef]
- Zhang, Y.; Qin, N.; Luo, Y.; Shen, H. Effects of different concentrations of salt and sugar on biogenic amines and quality changes of carp (Cyprinus carpio) during chilled storage. J. Sci. Food Agric. 2015, 95, 1157–1162. [Google Scholar] [CrossRef]
- Hao, R.; Liu, Y.; Sun, L.; Xia, L.; Jia, H.; Li, Q.; Pan, J. Sodium alginate coating with plant extract affected microbial communities, biogenic amine formation and quality properties of abalone (Haliotis discus hannai Ino) during chill storage. LWT-Food Sci. Technol. 2017, 81, 1–9. [Google Scholar] [CrossRef]
- Jamróz, E.; Kulawik, P.; Tkaczewska, J.; Guzik, P.; Zając, M.; Juszczak, L.; Krzyściak, P.; Turek, K. The effects of active double-layered furcellaran/gelatin hydrolysate film system with Ala-Tyr peptide on fresh Atlantic mackerel stored at −18 °C. Food Chem. 2021, 338, 127867. [Google Scholar] [CrossRef]
- De Abreu, D.P.; Losada, P.P.; Maroto, J.; Cruz, J. Natural antioxidant active packaging film and its effect on lipid damage in frozen blue shark (Prionace glauca). Innov. Food Sci. Emerg. Technol. 2011, 12, 50–55. [Google Scholar] [CrossRef]
- Boran, G.; Karaçam, H.; Boran, M. Changes in the quality of fish oils due to storage temperature and time. Food Chem. 2006, 98, 693–698. [Google Scholar] [CrossRef]
- Sun, X.; Guo, X.; Ji, M.; Wu, J.; Zhu, W.; Wang, J.; Cheng, C.; Chen, L.; Zhang, Q. Preservative effects of fish gelatin coating enriched with CUR/βCD emulsion on grass carp (Ctenopharyngodon idellus) fillets during storage at 4 C. Food Chem. 2019, 272, 643–652. [Google Scholar] [CrossRef]
- Chatkitanan, T.; Harnkarnsujarit, N. Development of nitrite compounded starch-based films to improve color and quality of vacuum-packaged pork. Food Packag. Shelf Life 2020, 25, 100521. [Google Scholar] [CrossRef]
- Oussalah, M.; Caillet, S.; Salmiéri, S.; Saucier, L.; Lacroix, M. Antimicrobial and antioxidant effects of milk protein-based film containing essential oils for the preservation of whole beef muscle. J. Agric. Food Chem. 2004, 52, 5598–5605. [Google Scholar] [CrossRef] [PubMed]


| Parameter | Compost | Double-Layered Films |
|---|---|---|
| pH | 8.12 ± 0.21 | 3.21 ± 0.25 |
| EC [mS/cm] | 2.88 ± 0.15 | 0.963 ± 0.05 |
| N [g/kg] | 2.443 ± 0.13 | 0.896 ± 0.04 |
| C [g/kg] | 30.65 ± 0.93 | 38.75 ± 0.99 |
| C:N | 12.54 ± 0.52 | 43.40 ± 1.50 |
| Solubility [%] | - | 72.79 ± 2.97 |
| Degree of dry matter loss after incubation [%] | 7.36 ± 5.01 | 84.95 ± 3.12 ** |
| Ash [%] | - | 4.95 ± 1.81 |
| Period | |||
|---|---|---|---|
| 0–112 h | 113–224 h | 224–336 h | |
| Control | Y = 0.6512 + 8.7823 R2 = 0.9681 | Y = 0.3023x + 41.982 R2 = 0.9794 | Y = 0.248x + 53.545 R2 = 0.9834 |
| Double-layered films | Y = 2.4205x + 7.7248 R2 = 0.9857 | Y = 0.6976x + 173.71 R2 = 0.9866 | Y = 0.3415x + 245.54 R2 = 0.9723 |
| Group/Day | TRPYP | PHEN | PUTRY | CADAWE | HISTAM | TYRAM | SPER | SPR | |
|---|---|---|---|---|---|---|---|---|---|
| Initial (day zero) | 0.30 ± 0.07 | 0.68 ± 0.16 | 3.50 ± 0.68 | 1.46 ± 0.56 | 6.26 ± 1.175 | 2.34 ± 0.50 | 2.34 ± 0.50 | 4.01 ± 0.65 | |
| LDPE | 3 | 1.08 b ± 0.12 | 0.62 b ± 0.03 | 2.83 b ± 0.40 | 29.83 b ± 7.67 | 26.36 a ± 4.17 | 3.91 b ± 1.09 | 2.12 b ± 0.06 | 2.94 a ± 0.34 |
| 6 | 3.16 b ± 0.19 | 5.42 b ± 0.36 | 9.00 b ± 1.00 | 61.06 a ± 2.24 | 86.48 b ± 13.50 | 23.34 b ± 1.27 | 2.08 a ± 0.23 | 3.42 b ± 0.21 | |
| 10 | 3.73 b ± 0.34 | 10.26 b ± 2.09 | 11.86 b ± 1.28 | 43.00 a ± 1.73 | 160.92 b ± 6.02 | 26.23 b ± 2.08 | 3.44 b ± 0.33 | 4.08 b ± 0.55 | |
| Double-layered films | 3 | 0.17 a ± 0.03 | 0.43 a ± 0.03 | 1.86 a ± 0.28 | 2.54 a ± 0.49 | 24.53 a ± 2.73 | 1.66 a ± 0.32 | 1.84 a ± 0.13 | 2.57 a ± 0.23 |
| 6 | 0.66 a ± 0.05 | 0.90 a ± 0.27 | 3.66 a ± 0.91 | 51.35 a ± 9.85 | 52.36 a ± 2.98 | 9.01 a ± 0.10 | 2.37 a ± 0.39 | 2.74 a ± 0.38 | |
| 10 | 1.26 a ± 0.25 | 4.26 a ± 0.25 | 4.54 a ± 0.4 | 44.66 a ± 1.58 | 87.44 a ± 3.49 | 18.07 a ± 2.43 | 1.87 a ± 0.41 | 2.81 a ± 0.62 | |
| Peroxide Value (Milliequivalents of Active Oxygen/kg Product) | ||||
|---|---|---|---|---|
| Day 0 | Day 3 | Day 6 | Day 10 | |
| LDPE | 7.47 ± 0.34 | 14.58 a ± 0.36 | 35.18 a ± 0.59 | 46.10 a ± 0.15 |
| Double-layered films | 14.51 a ± 0.14 | 27.55 a ± 2.40 | 35.70 a ± 1.79 | |
| Acid value [mg KOH/g] | ||||
| LDPE | 1.910.33 | 2.78 a ± 0.06 | 3.91 a ± 0.05 | 8.33 a ± 0.46 |
| Double-layered films | 2.21 a ± 0.29 | 3.83 a ± 1.11 | 4.05 a ± 1.38 | |
| TBARS [mg/kg] | ||||
| LDPE | 0.23 ± 0.02 | 0.18 a ± 0.03 | 0.80 a ± 0.05 | 0.34 a ± 0.12 |
| Double-layered films | 1.11 b ± 0.25 | 2.38 b ± 0.30 | 1.79 b ± 0.06 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tkaczewska, J.; Jamróz, E.; Guzik, P.; Kopeć, M. Attempt to Extend the Shelf-Life of Fish Products by Means of Innovative Double-Layer Active Biodegradable Films. Polymers 2022, 14, 1717. https://doi.org/10.3390/polym14091717
Tkaczewska J, Jamróz E, Guzik P, Kopeć M. Attempt to Extend the Shelf-Life of Fish Products by Means of Innovative Double-Layer Active Biodegradable Films. Polymers. 2022; 14(9):1717. https://doi.org/10.3390/polym14091717
Chicago/Turabian StyleTkaczewska, Joanna, Ewelina Jamróz, Paulina Guzik, and Michał Kopeć. 2022. "Attempt to Extend the Shelf-Life of Fish Products by Means of Innovative Double-Layer Active Biodegradable Films" Polymers 14, no. 9: 1717. https://doi.org/10.3390/polym14091717