Starch Nanocomposite Films: Migration Studies of Nanoparticles to Food Simulants and Bio-Disintegration in Soil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Nanocomposite Starch Films
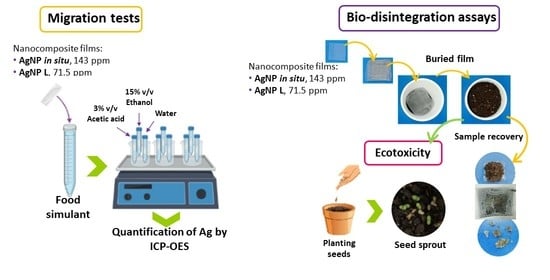
2.3. Migration Test
Quantification of Silver Content by ICP–OES
2.4. Mathematical Approach
2.5. Evaluation of the Films’ Bio-Disintegration
Ecotoxicity Estimation by Seeds Growth
2.6. Statistical Analysis
3. Results and Discussion
3.1. Nanocomposite Starch Films Characterization
3.2. Study of the Migration of Silver Nanoparticles from Nanocomposite Starch Films
Analysis of Silver Nanoparticles Release Kinetics
3.3. Evaluation of the Bio-Disintegration of Films in Soil
Compost Ecotoxicity Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cerro, D.; Bustos, G.; Villegas, C.; Buendia, N.; Truffa, G.; Godoy, M.P.; Rodríguez, F.; Rojas, A.; Galotto, M.J.; Constandil, L.; et al. Effect of Supercritical Incorporation of Cinnamaldehyde on Physical-Chemical Properties, Disintegration and Toxicity Studies of PLA/Lignin Nanocomposites. Int. J. Biol. Macromol. 2021, 167, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Tampau, A.; González-Martínez, C.; Chiralt, A. Biodegradability and Disintegration of Multilayer Starch Films with Electrospun PCL Fibres Encapsulating Carvacrol. Polym. Degrad. Stab. 2020, 173, 109100. [Google Scholar] [CrossRef]
- Nafchi, A.M.; Alias, A.K.; Mahmud, S.; Robal, M. Antimicrobial, Rheological, and Physicochemical Properties of Sago Starch Films Filled with Nanorod-Rich Zinc Oxide. J. Food Eng. 2012, 113, 511–519. [Google Scholar] [CrossRef]
- Abreu, A.S.; Oliveira, M.; De Sá, A.; Rodrigues, R.M.; Cerqueira, M.A.; Vicente, A.A.; Machado, A.V. Antimicrobial Nanostructured Starch Based Films for Packaging. Carbohydr. Polym. 2015, 129, 127–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guz, L.; Famá, L.; Candal, R.; Goyanes, S. Size Effect of ZnO Nanorods on Physicochemical Properties of Plasticized Starch Composites. Carbohydr. Polym. 2017, 157, 1611–1619. [Google Scholar] [CrossRef] [PubMed]
- Estevez-Areco, S.; Guz, L.; Famá, L.; Candal, R.; Goyanes, S. Bioactive Starch Nanocomposite Films with Antioxidant Activity and Enhanced Mechanical Properties Obtained by Extrusion Followed by Thermo-Compression. Food Hydrocoll. 2019, 96, 518–528. [Google Scholar] [CrossRef]
- Cheng, H.; Chen, L.; McClements, D.J.; Yang, T.; Zhang, Z.; Ren, F.; Miao, M.; Tian, Y.; Jin, Z. Starch-Based Biodegradable Packaging Materials: A Review of Their Preparation, Characterization and Diverse Applications in the Food Industry. Trends Food Sci. Technol. 2021, 114, 70–82. [Google Scholar] [CrossRef]
- Youssef, A.M.; El-Sayed, S.M. Bionanocomposites Materials for Food Packaging Applications: Concepts and Future Outlook. Carbohydr. Polym. 2018, 193, 19–27. [Google Scholar] [CrossRef]
- Mihindukulasuriya, S.D.F.; Lim, L.T. Nanotechnology Development in Food Packaging: A Review. Trends Food Sci. Technol. 2014, 40, 149–167. [Google Scholar] [CrossRef]
- Rhim, J.-W.W.; Park, H.-M.M.; Ha, C.-S.S. Bio-Nanocomposites for Food Packaging Applications. Prog. Polym. Sci. 2013, 38, 1629–1652. [Google Scholar] [CrossRef]
- Bumbudsanpharoke, N.; Ko, S. Nano-Food Packaging: An Overview of Market, Migration Research, and Safety Regulations. J. Food Sci. 2015, 80, R910–R923. [Google Scholar] [CrossRef] [PubMed]
- Souza, V.G.L.; Fernando, A.L. Nanoparticles in Food Packaging: Biodegradability and Potential Migration to Food—A Review. Food Packag. Shelf Life 2016, 8, 63–70. [Google Scholar] [CrossRef]
- Störmer, A.; Bott, J.; Kemmer, D.; Franz, R. Critical Review of the Migration Potential of Nanoparticles in Food Contact Plastics. Trends Food Sci. Technol. 2017, 63, 39–50. [Google Scholar] [CrossRef]
- Noonan, G.O.; Whelton, A.J.; Carlander, D.; Duncan, T. V Measurement Methods to Evaluate Engineered Nanomaterial Release from Food Contact Materials. Compr. Rev. Food Sci. Food Saf. 2014, 13, 679–692. [Google Scholar] [CrossRef]
- Picó, Y. Safety Assessment and Migration Tests. In Nanomaterials for Food Packaging: Materials, Processing Technologies, and Safety Issues; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 249–275. ISBN 9780323512718. [Google Scholar]
- Mohee, R.; Unmar, G.D.; Mudhoo, A.; Khadoo, P. Biodegradability of Biodegradable/Degradable Plastic Materials under Aerobic and Anaerobic Conditions. Waste Manag. 2008, 28, 1624–1629. [Google Scholar] [CrossRef]
- Kaur, K.; Jindal, R.; Maiti, M.; Mahajan, S. Studies on the Properties and Biodegradability of PVA/Trapa Natans Starch (N-St) Composite Films and PVA/N-St-g-Poly (EMA) Composite Films. Int. J. Biol. Macromol. 2019, 123, 826–836. [Google Scholar] [CrossRef]
- Ortega, F.; Giannuzzi, L.; Arce, V.B.; García, M.A. Active Composite Starch Films Containing Green Synthetized Silver Nanoparticles. Food Hydrocoll. 2017, 70, 152–162. [Google Scholar] [CrossRef]
- Ortega, F.; García, M.A.; Arce, V.B. Nanocomposite Films with Silver Nanoparticles Synthesized in Situ: Effect of Corn Starch Content. Food Hydrocoll. 2019, 97, 105200. [Google Scholar] [CrossRef]
- Ortega, F.; Arce, V.B.; Garcia, M.A. Nanocomposite Starch-Based Films Containing Silver Nanoparticles Synthesized with Lemon Juice as Reducing and Stabilizing Agent. Carbohydr. Polym. 2021, 252, 117208. [Google Scholar] [CrossRef]
- Ninago, M.D.; López, O.V.; Lencina, M.M.S.; García, M.A.; Andreucetti, N.A.; Ciolino, A.E.; Villar, M.A. Enhancement of Thermoplastic Starch Final Properties by Blending with Poly(ε-Caprolactone). Carbohydr. Polym. 2015, 134, 205–212. [Google Scholar] [CrossRef]
- Kim, M.H.; Kim, T.H.; Ko, J.A.; Ko, S.; Oh, J.M.; Park, H.J. Kinetic and Thermodynamic Studies of Silver Migration from Nanocomposites. J. Food Eng. 2019, 243, 1–8. [Google Scholar] [CrossRef]
- Leites Luchese, C.; Menegotto Frick, P.; Tessarol Cristina, I. Influence of the Incorporation Form of Waste from the Production of Orange Juice in the Properties of Cassava Starch-Based Films. Food Hydrocoll. 2021, 117, 106730. [Google Scholar] [CrossRef]
- Yu, Z.; Wang, W.; Kong, F.; Lin, M.; Mustapha, A. Cellulose Nanofibril/Silver Nanoparticle Composite as an Active Food Packaging System and Its Toxicity to Human Colon Cells. Int. J. Biol. Macromol. 2019, 129, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Di Rienzo, J.A.; Casanoves, F.; Balzarini, M.G.; González, L.; Tablada, M.; Robledo, C.W. InfoStat 2020. Grupo InfoStat, FCA, Universidad Nacional de Córdoba, Argentina (25, 01, 2022). Available online: http://www.infostat.com.ar (accessed on 31 March 2022).
- Crank, J. The Mathematics of Diffusion, 2nd ed.; Oxford University: Oxford, UK, 1979; ISBN 3804204422. [Google Scholar]
- Agustinelli, S.P.; Ciannamea, E.M.; Ruseckaite, R.A.; Martucci, J.F. Migration of Red Grape Extract Components and Glycerol from Soybean Protein Concentrate Active Films into Food Simulants. Food Hydrocoll. 2021, 120, 106955. [Google Scholar] [CrossRef]
- Ritger, P.L.; Peppas, N.A. A Simple Equation for Description of Solute Release II. Fickian and Anomalous Release from Swellable Devices. J. Control. Release 1987, 5, 37–42. [Google Scholar] [CrossRef]
- D’Souza, S.S.; Faraj, J.A.; DeLuca, P.P. A Model-Dependent Approach to Correlate Accelerated with Real-Time Release from Biodegradable Microspheres. AAPS PharmSciTech 2005, 6, E553–E564. [Google Scholar] [CrossRef] [Green Version]
- ASTM D5988-03; Standard Test Method for Determining Aerobic Biodegradation in Soil of Plastic Materials or Residual Plastic Materials after Composting. ASTM International: West Conshohocken, PA, USA, 2003.
- Gautam, N.; Kaur, I. Soil Burial Biodegradation Studies of Starch Grafted Polyethylene and Identification of Rhizobium Meliloti Therefrom. J. Environ. Chem. Ecotoxicol. 2013, 5, 147–158. [Google Scholar] [CrossRef]
- Kanmani, P.; Rhim, J.W. Physicochemical Properties of Gelatin/Silver Nanoparticle Antimicrobial Composite Films. Food Chem. 2014, 148, 162–169. [Google Scholar] [CrossRef]
- Cheviron, P.; Gouanvé, F.; Espuche, E. Starch/Silver Nanocomposite: Effect of Thermal Treatment Temperature on the Morphology, Oxygen and Water Transport Properties. Carbohydr. Polym. 2015, 134, 635–645. [Google Scholar] [CrossRef]
- Zhang, W.; Jiang, W. Antioxidant and Antibacterial Chitosan Film with Tea Polyphenols-Mediated Green Synthesis Silver Nanoparticle via a Novel One-Pot Method. Int. J. Biol. Macromol. 2020, 155, 1252–1261. [Google Scholar] [CrossRef]
- Cano, A.; Cháfer, M.; Chiralt, A.; González-Martínez, C. Development and Characterization of Active Films Based on Starch-PVA, Containing Silver Nanoparticles. Food Packag. Shelf Life 2016, 10, 16–24. [Google Scholar] [CrossRef]
- Papadopoulou, V.; Kosmidis, K.; Vlachou, M.; Macheras, P. On the Use of the Weibull Function for the Discernment of Drug Release Mechanisms. Int. J. Pharm. 2006, 309, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Dash, S.K.; Murthy, P.N.; Nath, L.; Chowdhury, P. Kinetic Modeling On Drug Release From Controlled Drug Delivery Systems. Pol. Pharm. Soc. 2010, 67, 217–223. [Google Scholar]
- Boostani, S.; Jafari, S.M. A Comprehensive Review on the Controlled Release of Encapsulated Food Ingredients; Fundamental Concepts to Design and Applications. Trends Food Sci. Technol. 2021, 109, 303–321. [Google Scholar] [CrossRef]
- Código Alimentario Argentino. Capítulo IV—Utensilios, Recipientes, Envases, Envolturas, Aparatos y Accesorios. Argentina, 2008. Available online: http://www.anmat.gov.ar/webanmat/codigoa/capitulo_iv_envases_actualiz_2008-12.pdf (accessed on 31 March 2022).
- Vilela Dias, M.; Sousa, M.M.; Lara, B.R.B.; de Azevedo, V.M.; de Fátima Ferreira Soares, N.; Borges, S.V.; Queiroz, F. Thermal and Morphological Properties and Kinetics of Diffusion of Antimicrobial Films on Food and a Simulant. Food Packag. Shelf Life 2018, 16, 15–22. [Google Scholar] [CrossRef]
- Lee, P.C.; Meisel, D. Adsorption and Surface-Enhanced Raman of Dyes on Silver and Gold Sols. J. Phys. Chem. 1982, 86, 3391–3395. [Google Scholar] [CrossRef]
- Heydari-Majd, M.; Ghanbarzadeh, B.; Shahidi-Noghabi, M.; Najafi, M.A.; Adun, P.; Ostadrahimid, A. Kinetic Release Study of Zinc from Polylactic Acid Based Nanocomposite into Food Simulants. Polym. Test. 2019, 76, 254–260. [Google Scholar] [CrossRef]
- Balzarini, M.G.; Gonzalez, L.; Tablada, M.; Casanoves, F.; Di Rienzo, J.A.; Robledo, C.W. Infostat. Manual Del Usuario; Editorial Brujas: Córdoba, Argentina, 2008. [Google Scholar]
- Reddy, N.; Yang, Y. Citric Acid Cross-Linking of Starch Films. Food Chem. 2010, 118, 702–711. [Google Scholar] [CrossRef] [Green Version]
- Shi, R.; Bi, J.; Zhang, Z.; Zhu, A.; Chen, D.; Zhou, X.; Zhang, L.; Tian, W. The Effect of Citric Acid on the Structural Properties and Cytotoxicity of the Polyvinyl Alcohol/Starch Films When Molding at High Temperature. Carbohydr. Polym. 2008, 74, 763–770. [Google Scholar] [CrossRef]
- Polat, S.; Fenercioğlu, H.; Güçlü, M. Effects of Metal Nanoparticles on the Physical and Migration Properties of Low Density Polyethylene Films. J. Food Eng. 2018, 229, 32–42. [Google Scholar] [CrossRef]
- Poças, F.; Franz, R. Chapter 10—Overview on European Regulatory Issues, Legislation, and EFSA Evaluations of Nanomaterials. In Nanomaterials for Food Packaging; Elsevier Inc.: Amsterdam, The Netherlands, 2018; Volume 1, pp. 277–300. ISBN 9780323512718. [Google Scholar]
- Luzi, F.; Fortunati, E.; Puglia, D.; Petrucci, R.; Kenny, J.M.; Torre, L. Study of Disintegrability in Compost and Enzymatic Degradation of PLA and PLA Nanocomposites Reinforced with Cellulose Nanocrystals Extracted from Posidonia Oceanica. Polym. Degrad. Stab. 2015, 121, 105–115. [Google Scholar] [CrossRef]
- Ramos, M.; Fortunati, E.; Peltzer, M.; Jimenez, A.; María Kenny, J.; Carmen Garrig os, M. Characterization and Disintegrability under Composting Conditions of PLA-Based Nanocomposite Films with Thymol and Silver Nanoparticles. Polym. Degrad. Stab. 2016, 132, 2–10. [Google Scholar] [CrossRef] [Green Version]
- Tai, N.L.; Adhikari, R.; Shanks, R.; Adhikari, B. Aerobic Biodegradation of Starch–Polyurethane Flexible Films under Soil Burial Condition: Changes in Physical Structure and Chemical Composition. Int. Biodeterior. Biodegrad. 2019, 145, 104793. [Google Scholar] [CrossRef]
- Zafar, U.; Houlden, A.; Robson, G.D. Fungal Communities Associated with the Biodegradation of Polyester Polyurethane Buried under Compost at Different Temperatures. Appl. Environ. Microbiol. 2013, 79, 7313–7324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Medina Jaramillo, C.; Gutiérrez, T.J.; Goyanes, S.; Bernal, C.; Famá, L. Biodegradability and Plasticizing Effect of Yerba Mate Extract on Cassava Starch Edible Films. Carbohydr. Polym. 2016, 151, 150–159. [Google Scholar] [CrossRef]
- Balaguer, M.P.; Villanova, J.; Cesar, G.; Gavara, R.; Hernandez-Munoz, P. Compostable Properties of Antimicrobial Bioplastics Based on Cinnamaldehyde Cross-Linked Gliadins. Chem. Eng. J. 2015, 262, 447–455. [Google Scholar] [CrossRef]
- Rychter, P.; Kawalec, M.; Sobota, M.; Kurcok, P.; Kowalczuk, M. Study of Aliphatic-Aromatic Copolyester Degradation in Sandy Soil and Its Ecotoxicological Impact. Biomacromolecules 2010, 11, 839–847. [Google Scholar] [CrossRef]







| Starch Film Sample | Thickness (μm) | WVP (10−10 g/m s Pa) | Mechanical Properties | ||
|---|---|---|---|---|---|
| E(%) | TS (MPa) | EM (MPa) | |||
| AgNP in situ | 97.3 ± 1.2 b | 1.38 ± 0.05 b | 32.5 ± 0.7 a | 5.8 ± 0.3 c | 15.2 ± 1.3 b |
| AgNP L | 102.7 ± 3.9 c | 0.63 ± 0.07 a | 40.0 ± 5.6 b | 4.0 ± 0.6 b | 14.2 ± 1.8 b |
| Control | 87.9 ± 5.0 a | 2.9 ± 0.1 c | 32.7 ± 0.8 a | 2.9 ± 0.2 a | 3.7 ± 0.6 a |
| Model | Water | 3% v/v Acetic Acid | 15% v/v Ethanol | |||
|---|---|---|---|---|---|---|
| AgNP L | AgNP In Situ | AgNP L | AgNP In Situ | AgNP L | AgNP In Situ | |
| Diffusion | ||||||
| D1 a (cm2/s) | 5.61 × 10−10 | 3.41 × 10−9 | 1.53 × 10−9 | 1.99 × 10−9 | 3.72 × 10−9 | 3.68 × 10−9 |
| R2 b | 0.91 | 0.98 | 0.92 | 0.98 | 0.98 | 0.98 |
| D2 c (cm2/s) | 1.33 × 10−7 | 1.84 × 10−9 | 1.02 × 10−9 | 1.14 × 10−9 | 1.77 × 10−9 | 3.57 × 10−10 |
| R2 b | 0.96 | 0.73 | 0.65 | 0.52 | 0.82 | 0.74 |
| Ritger and Peppas | ||||||
| n | 0.20 | 0.59 | 0.21 | 0.23 | 0.61 | 0.43 |
| k | 0.55 | 0.08 | 0.30 | 0.28 | 0.06 | 0.06 |
| MSE d | 8.55 × 10−6 | 5.5 × 10−4 | 1.9 × 10−3 | 1.4 × 10−3 | 1.4 × 10−3 | 3.8 × 10−5 |
| Weibull | ||||||
| a | 0.84 | 0.06 | 0.32 | 0.27 | 0.05 | 0.02 |
| b | 0.22 | 0.74 | 0.36 | 0.47 | 0.79 | 1.02 |
| MSE d | 2.2 × 10−3 | 1.2 × 10−3 | 0.01 | 2.4 × 10−3 | 2.1 × 10−3 | 2.8 × 10−3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortega, F.; Sobral, P.; Jios, J.L.; Arce, V.B.; García, M.A. Starch Nanocomposite Films: Migration Studies of Nanoparticles to Food Simulants and Bio-Disintegration in Soil. Polymers 2022, 14, 1636. https://doi.org/10.3390/polym14091636
Ortega F, Sobral P, Jios JL, Arce VB, García MA. Starch Nanocomposite Films: Migration Studies of Nanoparticles to Food Simulants and Bio-Disintegration in Soil. Polymers. 2022; 14(9):1636. https://doi.org/10.3390/polym14091636
Chicago/Turabian StyleOrtega, Florencia, Pablo Sobral, Jorge L. Jios, Valeria B. Arce, and María Alejandra García. 2022. "Starch Nanocomposite Films: Migration Studies of Nanoparticles to Food Simulants and Bio-Disintegration in Soil" Polymers 14, no. 9: 1636. https://doi.org/10.3390/polym14091636