Novel Mutations in Putative Nicotinic Acid Phosphoribosyltransferases of Mycobacterium tuberculosis and Their Effect on Protein Thermodynamic Properties
Abstract
:1. Introduction
2. Materials and Methods
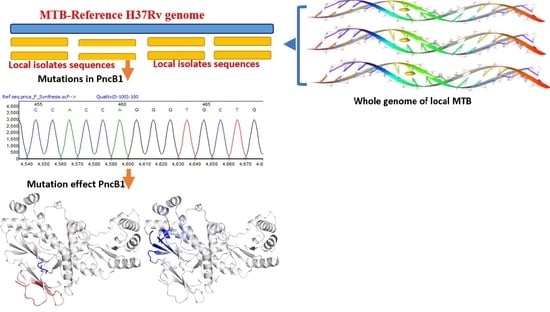
2.1. Whole-Genome Sequence Data Retrieval
2.2. Whole-Genome Sequence Analysis
2.3. Structure Modeling of pncB1
2.4. Ramachandran Plot of pncB1 Modelled Structure
2.5. Mutations Effect on pncB1 Stability
2.6. Secondary Structure Prediction of Wild Type and Mutant
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khan, M.T.; Malik, S.I.; Ali, S.; Sheed Khan, A.; Nadeem, T.; Zeb, M.T.; Masood, N.; Afzal, M.T. Prevalence of Pyrazinamide Resistance in Khyber Pakhtunkhwa, Pakistan. Microb. Drug Resist. Larchmt. N 2018, 24, 1417–1421. [Google Scholar] [CrossRef]
- Hou, X.-M.; Wang, C.-Y.; Gerwick, W.H.; Shao, C.-L. Marine Natural Products as Potential Anti-Tubercular Agents. Eur. J. Med. Chem. 2019, 165, 273–292. [Google Scholar] [CrossRef]
- Khan, M.T.; Kaushik, A.C.; Bhatti, A.I.; Zhang, Y.-J.; Zhang, S.; Wei, A.J.; Malik, S.I.; Wei, D.Q. Marine Natural Products and Drug Resistance in Latent Tuberculosis. Mar. Drugs 2019, 17, 549. [Google Scholar] [CrossRef] [Green Version]
- Ilin, A.I.; Kulmanov, M.E.; Korotetskiy, I.S.; Islamov, R.A.; Akhmetova, G.K.; Lankina, M.V.; Reva, O.N. Genomic Insight into Mechanisms of Reversion of Antibiotic Resistance in Multidrug Resistant Mycobacterium tuberculosis Induced by a Nanomolecular Iodine-Containing Complex FS-1. Front. Cell. Infect. Microbiol. 2017, 7, 151. [Google Scholar] [CrossRef]
- Durairaj, D.R.; Shanmughavel, P. In Silico Drug Design of Thiolactomycin Derivatives Against Mtb-KasA Enzyme to Inhibit Multidrug Resistance of Mycobacterium tuberculosis. Interdiscip. Sci. Comput. Life Sci. 2019, 11, 215–225. [Google Scholar] [CrossRef]
- Anand, R. Identification of Potential Antituberculosis Drugs Through Docking and Virtual Screening. Interdiscip. Sci. Comput. Life Sci. 2018, 10, 419–429. [Google Scholar] [CrossRef]
- Sharma, P.C.; Jain, A.; Yar, M.S.; Pahwa, R.; Singh, J.; Goel, S. Synthesis and Antibacterial Evaluation of Novel Analogs of Fluoroquinolones Annulated with 6-Substituted-2-Aminobenzothiazoles. Arab. J. Chem. 2015, 8, 671–677. [Google Scholar] [CrossRef] [Green Version]
- Boshoff, H.I.M.; Xu, X.; Tahlan, K.; Dowd, C.S.; Pethe, K.; Camacho, L.R.; Park, T.-H.; Yun, C.-S.; Schnappinger, D.; Ehrt, S.; et al. Biosynthesis and Recycling of Nicotinamide Cofactors in Mycobacterium tuberculosis. J. Biol. Chem. 2008, 283, 19329–19341. [Google Scholar] [CrossRef] [Green Version]
- Preiss, J.; Handler, P. Biosynthesis of Diphosphopyridine Nucleotide. I. Identification of Intermediates. J. Biol. Chem. 1958, 233, 488–492. [Google Scholar] [CrossRef]
- Via, L.E.; Lin, P.L.; Ray, S.M.; Carrillo, J.; Allen, S.S.; Eum, S.Y.; Taylor, K.; Klein, E.; Manjunatha, U.; Gonzales, J.; et al. Tuberculous Granulomas Are Hypoxic in Guinea Pigs, Rabbits, and Nonhuman Primates. Infect. Immun. 2008, 76, 2333–2340. [Google Scholar] [CrossRef] [Green Version]
- Voskuil, M.I.; Schnappinger, D.; Visconti, K.C.; Harrell, M.I.; Dolganov, G.M.; Sherman, D.R.; Schoolnik, G.K. Inhibition of Respiration by Nitric Oxide Induces a Mycobacterium tuberculosis Dormancy Program. J. Exp. Med. 2003, 198, 705–713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dahl, J.L.; Kraus, C.N.; Boshoff, H.I.M.; Doan, B.; Foley, K.; Avarbock, D.; Kaplan, G.; Mizrahi, V.; Rubin, H.; Barry, C.E. The Role of RelMtb-Mediated Adaptation to Stationary Phase in Long-Term Persistence of Mycobacterium tuberculosis in Mice. Proc. Natl. Acad. Sci. USA 2003, 100, 10026–10031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, M.; Ali, S.; Khan, A.; Sheed Ali, A.; Khan, A.; Kaushik, A.C.; Irfan, M.; Chinnasamy, S.; Zhang, S.; Zhang, Y.-J.; et al. Insight into the Drug Resistance Whole Genome of Mycobacterium tuberculosis Isolates from Khyber Pakhtunkhwa, Pakistan. Infect. Genet. Evol. 2021, 92, 104861. [Google Scholar] [CrossRef] [PubMed]
- Cole, S.T.; Brosch, R.; Parkhill, J.; Garnier, T.; Churcher, C.; Harris, D.; Gordon, S.V.; Eiglmeier, K.; Gas, S.; Barry, C.E.; et al. Deciphering the Biology of Mycobacterium tuberculosis from the Complete Genome Sequence. Nature 1998, 393, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Feuerriegel, S.; Schleusener, V.; Beckert, P.; Kohl, T.A.; Miotto, P.; Cirillo, D.M.; Cabibbe, A.M.; Niemann, S.; Fellenberg, K. PhyResSE: A Web Tool Delineating Mycobacterium tuberculosis Antibiotic Resistance and Lineage from Whole-Genome Sequencing Data. J. Clin. Microbiol. 2015, 53, 1908. [Google Scholar] [CrossRef] [Green Version]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef] [Green Version]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly Accurate Protein Structure Prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Ramakrishnan, C.; Ramachandran, G.N. Stereochemical Criteria for Polypeptide and Protein Chain Conformations. II. Allowed Conformations for a Pair of Peptide Units. Biophys. J. 1965, 5, 909–933. [Google Scholar] [CrossRef] [Green Version]
- Wlodawer, A. Stereochemistry and Validation of Macromolecular Structures. In Protein Crystallography; Methods in Molecular Biology; Humana Press: New York, NY, USA, 2017; Volume 1607, pp. 595–610. [Google Scholar] [CrossRef] [Green Version]
- Wiederstein, M.; Sippl, M.J. ProSA-Web: Interactive Web Service for the Recognition of Errors in Three-Dimensional Structures of Proteins. Nucleic Acids Res. 2007, 35 (Suppl. 2), W407–W410. Available online: https://prosa.services.came.sbg.ac.at/prosa.php (accessed on 8 April 2022).
- Rodrigues, C.H.; Pires, D.E.; Ascher, D.B. DynaMut: Predicting the Impact of Mutations on Protein Conformation, Flexibility and Stability. Nucleic Acids Res. 2018, 46, W350–W355. [Google Scholar] [CrossRef]
- McGuffin, L.J.; Bryson, K.; Jones, D.T. The PSIPRED Protein Structure Prediction Server. Bioinformatics 2000, 16, 404–405. [Google Scholar] [CrossRef]
- Coll, F.; Phelan, J.; Hill-Cawthorne, G.A.; Nair, M.B.; Mallard, K.; Ali, S.; Abdallah, A.M.; Alghamdi, S.; Alsomali, M.; Ahmed, A.O.; et al. Genome-Wide Analysis of Multi- and Extensively Drug-Resistant Mycobacterium tuberculosis. Nat. Genet. 2018, 50, 307–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zwart, M.P.; Schenk, M.F.; Hwang, S.; Koopmanschap, B.; de Lange, N.; van de Pol, L.; Nga, T.T.T.; Szendro, I.G.; Krug, J.; de Visser, J.A.G.M. Unraveling the Causes of Adaptive Benefits of Synonymous Mutations in TEM-1 β-Lactamase. Heredity 2018, 121, 406–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plotkin, J.B.; Kudla, G. Synonymous but Not the Same: The Causes and Consequences of Codon Bias. Nat. Rev. Genet. 2011, 12, 32–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lind, P.A.; Berg, O.G.; Andersson, D.I. Mutational Robustness of Ribosomal Protein Genes. Science 2010, 330, 825–827. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, P.; de la Iglesia, F.; Elena, S.F. Distribution of Fitness and Virulence Effects Caused by Single-Nucleotide Substitutions in Tobacco Etch Virus. J. Virol. 2007, 81, 12979–12984. [Google Scholar] [CrossRef] [Green Version]
- Gustafsson, C.; Govindarajan, S.; Minshull, J. Codon Bias and Heterologous Protein Expression. Trends Biotechnol. 2004, 22, 346–353. [Google Scholar] [CrossRef]
- Zhang, F.; Saha, S.; Shabalina, S.A.; Kashina, A. Differential Arginylation of Actin Isoforms Is Regulated by Coding Sequence-Dependent Degradation. Science 2010, 329, 1534–1537. [Google Scholar] [CrossRef] [Green Version]
- Doss, C.G.P.; Rajith, B.; Garwasis, N.; Mathew, P.R.; Raju, A.S.; Apoorva, K.; William, D.; Sadhana, N.R.; Himani, T.; Dike, I.P. Screening of Mutations Affecting Protein Stability and Dynamics of FGFR1—A Simulation Analysis. Appl. Transl. Genom. 2012, 1, 37–43. [Google Scholar] [CrossRef] [Green Version]





| Position | Nucleotide Change | Type of Mutation | Amino Acid Change | |
|---|---|---|---|---|
| 1499213 | T | Del | * GAP | |
| 1499214 | C | Del | GAP | |
| 1499215 | A | Del | GAP | |
| 1499216 | G | Del | GAP | |
| 1499217 | G | Del | GAP | |
| 1499218 | C | Del | GAP | |
| 1499219 | C | Del | GAP | |
| 1499220 | G | Del | GAP | |
| 1499221 | G | SNP | A | * Pro447Ser (ccg/Tcg) |
| 1499274 | C | SNP | G | Gly429Ala (ggc/gCc) |
| 1500201 | G | SNP | A | Ser120Leu (tca/tTa) |
| 1499702 | C | SNP | G | * Arg286Arg (cgg/cgC) |
| 1500181 | C | SNP | T | * Gly127Ser (ggc/Agc) |
| Sample | Position | Wild Type | Mutant | Amino Acid Change |
|---|---|---|---|---|
| 737 | 666631 | A | G | Phe204Phe (ttt/ttC) |
| 741 | 666631 | A | G | Phe204Phe (ttt/ttC) |
| 752 | 666742 | C | T | Ala167Ala (gcg/gcA) |
| 754 | 666631 | A | G | Phe204Phe (ttt/ttC) |
| 767 | 666631 | A | G | Phe204Phe (ttt/ttC) |
| 770 | 666631 | A | G | Phe204Phe (ttt/ttC) |
| 790 | 666631 | A | G | Phe204Phe (ttt/ttC) |
| 797 | 666742 | C | T | Ala167Ala (gcg/gcA) |
| 801 | 666631 | A | G | Phe204Phe (ttt/ttC) |
| 802 | 666631 | A | G | Phe204Phe (ttt/ttC) |
| * ERR2510337 | 666275 | G | A | # Ala323Val (gcg/gTg) |
| * ERR2510358 | 666387 | A | C | # Phe286Val (ttc/Gtc) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.-J.; Khan, M.T.; Lodhi, M.S.; Al-Amrah, H.; Alrdahe, S.S.; Alatawi, H.A.; Darwish, D.B.E. Novel Mutations in Putative Nicotinic Acid Phosphoribosyltransferases of Mycobacterium tuberculosis and Their Effect on Protein Thermodynamic Properties. Polymers 2022, 14, 1623. https://doi.org/10.3390/polym14081623
Zhang Y-J, Khan MT, Lodhi MS, Al-Amrah H, Alrdahe SS, Alatawi HA, Darwish DBE. Novel Mutations in Putative Nicotinic Acid Phosphoribosyltransferases of Mycobacterium tuberculosis and Their Effect on Protein Thermodynamic Properties. Polymers. 2022; 14(8):1623. https://doi.org/10.3390/polym14081623
Chicago/Turabian StyleZhang, Yu-Juan, Muhammad Tahir Khan, Madeeha Shahzad Lodhi, Hadba Al-Amrah, Salma Saleh Alrdahe, Hanan Ali Alatawi, and Doaa Bahaa Eldin Darwish. 2022. "Novel Mutations in Putative Nicotinic Acid Phosphoribosyltransferases of Mycobacterium tuberculosis and Their Effect on Protein Thermodynamic Properties" Polymers 14, no. 8: 1623. https://doi.org/10.3390/polym14081623