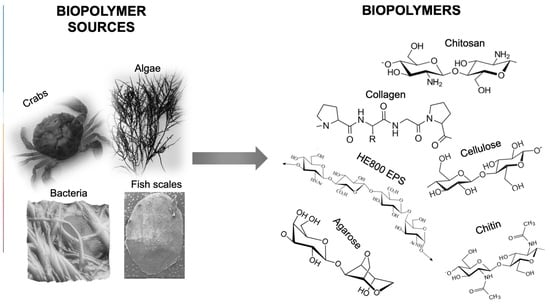
Contributions of Women in Recent Research on Biopolymer Science
Abstract
:1. Introduction
2. Advances in Biopolymer Research: Examples from Well-Known Biopolymers
2.1. Agar
2.2. Chitin and Chitosan
2.3. Cellulose
2.3.1. Cellulose and Nanocellulose
2.3.2. Bacterial Cellulose

2.4. Collagen
3. Discovery of Novel Marine Biopolymers and Carbohydrate-Active Enzymes
3.1. Unraveling the Extensive Potential of Polysaccharides from (Marine) Microscopic Life
3.2. Increasing Importance of Marine Carbohydrate-Active Enzymes
4. Conclusions and Perspectives
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Fernández-Marín, R.; Fernandes, S.C.M.; Sánchez, M.Á.A.; Labidi, J. Halochromic and Antioxidant Capacity of Smart Films of Chitosan/Chitin Nanocrystals with Curcuma Oil and Anthocyanins. Food Hydrocoll. 2022, 123, 107119. [Google Scholar] [CrossRef]
- Fernández-Marín, R.; Hernández-Ramos, F.; Salaberria, A.M.; Andrés, M.Á.; Labidi, J.; Fernandes, S.C.M. Eco-Friendly Isolation and Characterization of Nanochitin from Different Origins by Microwave Irradiation: Optimization Using Response Surface Methodology. Int. J. Biol. Macromol. 2021, 186, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Marín, R.; Mujtaba, M.; Cansaran-Duman, D.; Ben Salha, G.; Andrés Sánchez, M.Á.; Labidi, J.; Fernandes, S.C.M. Effect of Deterpenated Origanum majorana L. Essential Oil on the Physicochemical and Biological Properties of Chitosan/β-Chitin Nanofibers Nanocomposite Films. Polymers 2021, 13, 1507. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Marín, R.; Labidi, J.; Andrés, M.Á.; Fernandes, S.C.M. Using α-Chitin Nanocrystals to Improve the Final Properties of Poly (Vinyl Alcohol) Films with Origanum vulgare Essential Oil. Polym. Degrad. Stab. 2020, 179, 109227. [Google Scholar] [CrossRef]
- Gutierrez, J.; Fernandes, S.C.M.; Mondragon, I.; Tercjak, A. Multifunctional Hybrid Nanopapers Based on Bacterial Cellulose and Sol–Gel Synthesized Titanium/Vanadium Oxide Nanoparticles. Cellulose 2013, 20, 1301–1311. [Google Scholar] [CrossRef]
- Fernandes, S.; Freire, C.S.R.; Neto, C.P.; Gandini, A. The Bulk Oxypropylation of Chitin and Chitosan and the Characterization of the Ensuing Polyols. Green Chem. 2008, 10, 93–97. [Google Scholar] [CrossRef]
- Gutierrez, J.; Fernandes, S.C.M.; Mondragon, I.; Tercjak, A. Conductive Photoswitchable Vanadium Oxide Nanopaper Based on Bacterial Cellulose. ChemSusChem 2012, 5, 2323–2327. [Google Scholar] [CrossRef] [PubMed]
- Akoumany, K.; Zykwinska, A.; Sinquin, C.; Marchand, L.; Fanuel, M.; Ropartz, D.; Rogniaux, H.; Pipelier, M.; Delbarre-Ladrat, C.; Colliec-Jouault, S. Characterization of New Oligosaccharides Obtained by An Enzymatic Cleavage of the Exopolysaccharide Produced by the Deep-Sea Bacterium Alteromonas infernus Using Its Cell Extract. Molecules 2019, 24, 3441. [Google Scholar] [CrossRef]
- Zykwinska, A.; Berre, L.T.-L.; Sinquin, C.; Ropartz, D.; Rogniaux, H.; Colliec-Jouault, S.; Delbarre-Ladrat, C. Enzymatic Depolymerization of the GY785 Exopolysaccharide Produced by the Deep-Sea Hydrothermal Bacterium Alteromonas infernus: Structural Study and Enzyme Activity Assessment. Carbohydr. Polym. 2018, 188, 101–107. [Google Scholar] [CrossRef]
- Schultz-Johansen, M.; Bech, P.K.; Hennessy, R.C.; Glaring, M.A.; Barbeyron, T.; Czjzek, M.; Stougaard, P. A Novel Enzyme Portfolio for Red Algal Polysaccharide Degradation in the Marine Bacterium Paraglaciecola hydrolytica S66T Encoded in a Sizeable Polysaccharide Utilization Locus. Front. Microbiol. 2018, 9, 839. [Google Scholar] [CrossRef]
- Hesse, W.; Gröschel, D.H.M. Walther and Angelina Hesse-Early Contributors to Bacteriology In an Unassuming Way,. They Moved Agar from the Kitchen to the Lab, Revolutionizing Bacteriology. ASM News 1992, 58, 425–428. [Google Scholar]
- Farr, W.K. Structure and Composition of Plant Cell Membranes. Nature 1940, 146, 153–155. [Google Scholar] [CrossRef]
- George, A.; Sanjay, M.R.; Srisuk, R.; Parameswaranpillai, J.; Siengchin, S. A Comprehensive Review on Chemical Properties and Applications of Biopolymers and Their Composites. Int. J. Biol. Macromol. 2020, 154, 329–338. [Google Scholar] [CrossRef]
- Gheorghita, R.; Anchidin-Norocel, L.; Filip, R.; Dimian, M.; Covasa, M. Applications of Biopolymers for Drugs and Probiotics Delivery. Polymers 2021, 13, 2729. [Google Scholar] [CrossRef]
- Payen, A. Sur la gélose et les nids de salangane. CR Acad. Sci. Paris 1859, 1859, 521–530. (In French) [Google Scholar]
- Koch, R. “Die Aetiologie der Tuberculose” (The etiology of tuberculosis). Berl. Klin. Wochenschr. 1882, 19, 221–230. (In German) [Google Scholar] [CrossRef]
- Lee, W.-K.; Lim, Y.-Y.; Leow, A.T.-C.; Namasivayam, P.; Ong Abdullah, J.; Ho, C.-L. Biosynthesis of Agar in Red Seaweeds: A Review. Carbohydr. Polym. 2017, 164, 23–30. [Google Scholar] [CrossRef]
- Holdt, S.L.; Kraan, S. Bioactive Compounds in Seaweed: Functional Food Applications and Legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Torres, M.D.; Flórez-Fernández, N.; Domínguez, H. Integral Utilization of Red Seaweed for Bioactive Production. Mar. Drugs 2019, 17, 314. [Google Scholar] [CrossRef]
- Bixler, H.J.; Porse, H. A Decade of Change in the Seaweed Hydrocolloids Industry. J. Appl. Phycol. 2011, 23, 321–335. [Google Scholar] [CrossRef]
- Martínez-Sanz, M.; Gómez-Mascaraque, L.G.; Ballester, A.R.; Martínez-Abad, A.; Brodkorb, A.; López-Rubio, A. Production of Unpurified Agar-Based Extracts from Red Seaweed Gelidium Sesquipedale by Means of Simplified Extraction Protocols. Algal Res. 2019, 38, 101420. [Google Scholar] [CrossRef]
- Martínez-Sanz, M.; Gomez-Barrio, L.P.; Zhao, M.; Tiwari, B.; Knutsen, S.H.; Ballance, S.; Zobel, H.K.; Nilsson, A.E.; Krewer, C.; Östergren, K.; et al. Alternative Protocols for the Production of More Sustainable Agar-Based Extracts from Gelidium Sesquipedale. Algal Res. 2021, 55, 102254. [Google Scholar] [CrossRef]
- Trigueros, E.; Sanz, M.T.; Alonso-Riaño, P.; Beltrán, S.; Ramos, C.; Melgosa, R. Recovery of the Protein Fraction with High Antioxidant Activity from Red Seaweed Industrial Solid Residue after Agar Extraction by Subcritical Water Treatment. J. Appl. Phycol. 2021, 33, 1181–1194. [Google Scholar] [CrossRef]
- Sousa, A.M.M.; Alves, V.D.; Morais, S.; Delerue-Matos, C.; Gonçalves, M.P. Agar Extraction from Integrated Multitrophic Aquacultured Gracilaria vermiculophylla: Evaluation of a Microwave-Assisted Process Using Response Surface Methodology. Bioresour. Technol. 2010, 101, 3258–3267. [Google Scholar] [CrossRef]
- Sousa, A.M.M.; Morais, S.; Abreu, M.H.; Pereira, R.; Sousa-Pinto, I.; Cabrita, E.J.; Delerue-Matos, C.; Gonçalves, M.P. Structural, Physical, and Chemical Modifications Induced by Microwave Heating on Native Agar-like Galactans. J. Agric. Food Chem. 2012, 60, 4977–4985. [Google Scholar] [CrossRef]
- Sousa, A.M.M.; Borges, J.; Silva, A.F.; Gonçalves, M.P. Influence of the Extraction Process on the Rheological and Structural Properties of Agars. Carbohydr. Polym. 2013, 96, 163–171. [Google Scholar] [CrossRef]
- Sousa, A.M.M.; Borges, J.; Silva, F.; Ramos, A.M.; Cabrita, E.J.; Gonçalves, M.P. Shaping the Molecular Assemblies of Native and Alkali-Modified Agars in Dilute and Concentrated Aqueous Media via Microwave-Assisted Extraction. Soft Matter 2013, 9, 3131. [Google Scholar] [CrossRef]
- Sousa, A.M.M.; Souza, H.K.S.; Uknalis, J.; Liu, S.-C.; Gonçalves, M.P.; Liu, L. Electrospinning of Agar/PVA Aqueous Solutions and Its Relation with Rheological Properties. Carbohydr. Polym. 2015, 115, 348–355. [Google Scholar] [CrossRef]
- Guerrero, P.; Garrido, T.; Leceta, I.; De la Caba, K. Films Based on Proteins and Polysaccharides: Preparation and Physical–Chemical Characterization. Eur. Polym. J. 2013, 49, 3713–3721. [Google Scholar] [CrossRef]
- Guerrero, P.; Etxabide, A.; Leceta, I.; Peñalba, M.; De la Caba, K. Extraction of Agar from Gelidium sesquipedale (Rodhopyta) and Surface Characterization of Agar Based Films. Carbohydr. Polym. 2014, 99, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Garrido, T.; Etxabide, A.; Guerrero, P.; De la Caba, K. Characterization of Agar/Soy Protein Biocomposite Films: Effect of Agar on the Extruded Pellets and Compression Moulded Films. Carbohydr. Polym. 2016, 151, 408–416. [Google Scholar] [CrossRef]
- Sousa, A.M.M.; Gonçalves, M.P. Strategies to Improve the Mechanical Strength and Water Resistance of Agar Films for Food Packaging Applications. Carbohydr. Polym. 2015, 132, 196–204. [Google Scholar] [CrossRef]
- Sousa, A.M.M.; Souza, H.K.S.; Liu, L.; Gonçalves, M.P. Alternative Plasticizers for the Production of Thermo-Compressed Agar Films. Int. J. Biol. Macromol. 2015, 76, 138–145. [Google Scholar] [CrossRef]
- Martínez-Sanz, M.; Martínez-Abad, A.; López-Rubio, A. Cost-Efficient Bio-Based Food Packaging Films from Unpurified Agar-Based Extracts. Food Packag. Shelf Life 2019, 21, 100367. [Google Scholar] [CrossRef]
- De Oliveira, J.P.; Bruni, G.P.; Fabra, M.J.; Da Rosa Zavareze, E.; López-Rubio, A.; Martínez-Sanz, M. Development of Food Packaging Bioactive Aerogels through the Valorization of Gelidium sesquipedale Seaweed. Food Hydrocoll. 2019, 89, 337–350. [Google Scholar] [CrossRef]
- Martínez-Sanz, M.; Ström, A.; Lopez-Sanchez, P.; Knutsen, S.H.; Ballance, S.; Zobel, H.K.; Sokolova, A.; Gilbert, E.P.; López-Rubio, A. Advanced Structural Characterisation of Agar-Based Hydrogels: Rheological and Small Angle Scattering Studies. Carbohydr. Polym. 2020, 236, 115655. [Google Scholar] [CrossRef]
- Alehosseini, A.; Gomez del Pulgar, E.-M.; Fabra, M.J.; Gómez-Mascaraque, L.G.; Benítez-Páez, A.; Sarabi-Jamab, M.; Ghorani, B.; Lopez-Rubio, A. Agarose-Based Freeze-Dried Capsules Prepared by the Oil-Induced Biphasic Hydrogel Particle Formation Approach for the Protection of Sensitive Probiotic Bacteria. Food Hydrocoll. 2019, 87, 487–496. [Google Scholar] [CrossRef]
- Periayah, M.; Halim, A.; Saad, A.M. Chitosan: A Promising Marine Polysaccharide for Biomedical Research. Phcog. Rev. 2016, 10, 39. [Google Scholar] [CrossRef]
- Darmon, S.E.; Rudall, K.M. Infra-Red and X-ray Studies of Chitin. Discuss. Faraday Soc. 1950, 9, 251. [Google Scholar] [CrossRef]
- Jin, T.; Liu, T.; Lam, E.; Moores, A. Chitin and Chitosan on the Nanoscale. Nanoscale Horiz. 2021, 6, 505–542. [Google Scholar] [CrossRef]
- Wan, A.C.A.; Tai, B.C.U. CHITIN—A Promising Biomaterial for Tissue Engineering and Stem Cell Technologies. Biotechnol. Adv. 2013, 31, 1776–1785. [Google Scholar] [CrossRef]
- Muxika, A.; Etxabide, A.; Uranga, J.; Guerrero, P.; De la Caba, K. Chitosan as a Bioactive Polymer: Processing, Properties and Applications. Int. J. Biol. Macromol. 2017, 105, 1358–1368. [Google Scholar] [CrossRef]
- Hajji, S.; Younes, I.; Ghorbel-Bellaaj, O.; Hajji, R.; Rinaudo, M.; Nasri, M.; Jellouli, K. Structural Differences between Chitin and Chitosan Extracted from Three Different Marine Sources. Int. J. Biol. Macromol. 2014, 65, 298–306. [Google Scholar] [CrossRef]
- Claverie, M.; McReynolds, C.; Petitpas, A.; Thomas, M.; Fernandes, S.C.M. Marine-Derived Polymeric Materials and Biomimetics: An Overview. Polymers 2020, 12, 1002. [Google Scholar] [CrossRef]
- Tolaimate, A.; Rhazi, M.; Alagui, A.; Desbrieres, J.; Rinaudo, M. Valorization of Waste Products from Fishing Industry by Production of Chitin and Chitosan. Phys. Chem. News 2008, 42, 120–127. [Google Scholar]
- Hajji, S.; Younes, I.; Rinaudo, M.; Jellouli, K.; Nasri, M. Characterization and In Vitro Evaluation of Cytotoxicity, Antimicrobial and Antioxidant Activities of Chitosans Extracted from Three Different Marine Sources. Appl. Biochem. Biotechnol. 2015, 177, 18–35. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and Chitosan: Properties and Applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Younes, I.; Ghorbel-Bellaaj, O.; Nasri, R.; Chaabouni, M.; Rinaudo, M.; Nasri, M. Chitin and Chitosan Preparation from Shrimp Shells Using Optimized Enzymatic Deproteinization. Process Biochem. 2012, 47, 2032–2039. [Google Scholar] [CrossRef]
- Younes, I.; Hajji, S.; Frachet, V.; Rinaudo, M.; Jellouli, K.; Nasri, M. Chitin Extraction from Shrimp Shell Using Enzymatic Treatment. Antitumor, Antioxidant and Antimicrobial Activities of Chitosan. Int. J. Biol. Macromol. 2014, 69, 489–498. [Google Scholar] [CrossRef]
- Younes, I.; Hajji, S.; Rinaudo, M.; Chaabouni, M.; Jellouli, K.; Nasri, M. Optimization of Proteins and Minerals Removal from Shrimp Shells to Produce Highly Acetylated Chitin. Int. J. Biol. Macromol. 2016, 84, 246–253. [Google Scholar] [CrossRef]
- Ifuku, S.; Saimoto, H. Chitin Nanofibers: Preparations, Modifications, and Applications. Nanoscale 2012, 4, 3308–3318. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, S.; Brooks, A.K.; Yadavalli, V.K. Nature-Derived Materials for the Fabrication of Functional Biodevices. Mater. Today Bio 2020, 7, 100065. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, S.C.M.; Freire, C.S.R.; Silvestre, A.J.D.; Neto, C.P.; Gandini, A.; Desbriéres, J.; Blanc, S.; Ferreira, R.A.S.; Carlos, L.D. A Study of the Distribution of Chitosan onto and within a Paper Sheet Using a Fluorescent Chitosan Derivative. Carbohydr. Polym. 2009, 78, 760–766. [Google Scholar] [CrossRef]
- Fernandes, S.C.M.; Freire, C.S.R.; Silvestre, A.J.D.; Desbrières, J.; Gandini, A.; Neto, C.P. Production of Coated Papers with Improved Properties by Using a Water-Soluble Chitosan Derivative. Ind. Eng. Chem. Res. 2010, 49, 6432–6438. [Google Scholar] [CrossRef]
- Zubillaga, V.; Salaberria, A.M.; Palomares, T.; Alonso-Varona, A.; Kootala, S.; Labidi, J.; Fernandes, S.C.M. Chitin Nanoforms Provide Mechanical and Topological Cues to Support Growth of Human Adipose Stem Cells in Chitosan Matrices. Biomacromolecules 2018, 19, 3000–3012. [Google Scholar] [CrossRef]
- Zubillaga, V.; Alonso-Varona, A.; Fernandes, S.C.M.; Salaberria, A.M.; Palomares, T. Adipose-Derived Mesenchymal Stem Cell Chondrospheroids Cultured in Hypoxia and a 3D Porous Chitosan/Chitin Nanocrystal Scaffold as a Platform for Cartilage Tissue Engineering. Int. J. Mol. Sci. 2020, 21, 1004. [Google Scholar] [CrossRef]
- Zhu, K.; Duan, J.; Guo, J.; Wu, S.; Lu, A.; Zhang, L. High-Strength Films Consisted of Oriented Chitosan Nanofibers for Guiding Cell Growth. Biomacromolecules 2017, 18, 3904–3912. [Google Scholar] [CrossRef]
- Gao, H.; Zhong, Z.; Xia, H.; Hu, Q.; Ye, Q.; Wang, Y.; Chen, L.; Du, Y.; Shi, X.; Zhang, L. Construction of Cellulose Nanofibers/Quaternized Chitin/Organic Rectorite Composites and Their Application as Wound Dressing Materials. Biomater. Sci. 2019, 7, 2571–2581. [Google Scholar] [CrossRef]
- Liu, H.; Liu, J.; Qi, C.; Fang, Y.; Zhang, L.; Zhuo, R.; Jiang, X. Thermosensitive Injectable In-Situ Forming Carboxymethyl Chitin Hydrogel for Three-Dimensional Cell Culture. Acta Biomater. 2016, 35, 228–237. [Google Scholar] [CrossRef]
- Yang, X.; Yang, H.; Jiang, X.; Yang, B.; Zhu, K.; Lai, N.C.-H.; Huang, C.; Chang, C.; Bian, L.; Zhang, L. Injectable Chitin Hydrogels with Self-Healing Property and Biodegradability as Stem Cell Carriers. Carbohydr. Polym. 2021, 256, 117574. [Google Scholar] [CrossRef]
- Wu, S.; Duan, B.; Lu, A.; Wang, Y.; Ye, Q.; Zhang, L. Biocompatible Chitin/Carbon Nanotubes Composite Hydrogels as Neuronal Growth Substrates. Carbohydr. Polym. 2017, 174, 830–840. [Google Scholar] [CrossRef]
- Huang, W.; Wang, Y.; Huang, Z.; Wang, X.; Chen, L.; Zhang, Y.; Zhang, L. On-Demand Dissolvable Self-Healing Hydrogel Based on Carboxymethyl Chitosan and Cellulose Nanocrystal for Deep Partial Thickness Burn Wound Healing. ACS Appl. Mater. Interfaces 2018, 10, 41076–41088. [Google Scholar] [CrossRef]
- Liang, X.; Wang, X.; Xu, Q.; Lu, Y.; Zhang, Y.; Xia, H.; Lu, A.; Zhang, L. Rubbery Chitosan/Carrageenan Hydrogels Constructed through an Electroneutrality System and Their Potential Application as Cartilage Scaffolds. Biomacromolecules 2018, 19, 340–352. [Google Scholar] [CrossRef]
- Pei, X.; Deng, Y.; Li, Y.; Huang, Y.; Yuan, K.; Lee, J.-F.; Chan, T.-S.; Zhou, J.; Lei, A.; Zhang, L. Size-Controllable Ultrafine Palladium Nanoparticles Immobilized on Calcined Chitin Microspheres as Efficient and Recyclable Catalysts for Hydrogenation. Nanoscale 2018, 10, 14719–14725. [Google Scholar] [CrossRef]
- Pei, X.; Li, Y.; Deng, Y.; Lu, L.; Li, W.; Shi, R.; Lei, A.; Zhang, L. Chitin Microsphere Supported Pd Nanoparticles as an Efficient and Recoverable Catalyst for CO Oxidation and Heck Coupling Reaction. Carbohydr. Polym. 2021, 251, 117020. [Google Scholar] [CrossRef]
- Wu, S.; Duan, B.; Zeng, X.; Lu, A.; Xu, X.; Wang, Y.; Ye, Q.; Zhang, L. Construction of Blood Compatible Lysine-Immobilized Chitin/Carbon Nanotube Microspheres and Potential Applications for Blood Purified Therapy. J. Mater. Chem. B 2017, 5, 2952–2963. [Google Scholar] [CrossRef]
- Su, X.; Tan, M.; Duan, B.; Cai, J.; Jiang, W.; Zhang, L. Hierarchical Microspheres with Macropores Fabricated from Chitin as 3D Cell Culture. J. Mater. Chem. B 2019, 7, 5190–5198. [Google Scholar] [CrossRef]
- Sacco, P.; Furlani, F.; Cok, M.; Travan, A.; Borgogna, M.; Marsich, E.; Paoletti, S.; Donati, I. Boric Acid Induced Transient Cross-Links in Lactose-Modified Chitosan (Chitlac). Biomacromolecules 2017, 18, 4206–4213. [Google Scholar] [CrossRef]
- Vecchies, F.; Sacco, P.; Decleva, E.; Menegazzi, R.; Porrelli, D.; Donati, I.; Turco, G.; Paoletti, S.; Marsich, E. Complex Coacervates between a Lactose-Modified Chitosan and Hyaluronic Acid as Radical-Scavenging Drug Carriers. Biomacromolecules 2018, 19, 3936–3944. [Google Scholar] [CrossRef]
- Furlani, F.; Sacco, P.; Scognamiglio, F.; Asaro, F.; Travan, A.; Borgogna, M.; Marsich, E.; Cok, M.; Paoletti, S.; Donati, I. Nucleation, Reorganization and Disassembly of an Active Network from Lactose-Modified Chitosan Mimicking Biological Matrices. Carbohydr. Polym. 2019, 208, 451–456. [Google Scholar] [CrossRef]
- Pizzolitto, C.; Cok, M.; Asaro, F.; Scognamiglio, F.; Marsich, E.; Lopez, F.; Donati, I.; Sacco, P. On the Mechanism of Genipin Binding to Primary Amines in Lactose-Modified Chitosan at Neutral PH. Int. J. Mol. Sci. 2020, 21, 6831. [Google Scholar] [CrossRef]
- Danti, S.; Trombi, L.; Fusco, A.; Azimi, B.; Lazzeri, A.; Morganti, P.; Coltelli, M.-B.; Donnarumma, G. Chitin Nanofibrils and Nanolignin as Functional Agents in Skin Regeneration. Int. J. Mol. Sci. 2019, 20, 2669. [Google Scholar] [CrossRef]
- Fan, Y.; Saito, T.; Isogai, A. Preparation of Chitin Nanofibers from Squid Pen β-Chitin by Simple Mechanical Treatment under Acid Conditions. Biomacromolecules 2008, 9, 1919–1923. [Google Scholar] [CrossRef]
- Jiang, J.; Ye, W.; Yu, J.; Fan, Y.; Ono, Y.; Saito, T.; Isogai, A. Chitin Nanocrystals Prepared by Oxidation of α-Chitin Using the O2/Laccase/TEMPO System. Carbohydr. Polym. 2018, 189, 178–183. [Google Scholar] [CrossRef]
- Fan, Y.; Saito, T.; Isogai, A. Chitin Nanocrystals Prepared by TEMPO-Mediated Oxidation of α-Chitin. Biomacromolecules 2008, 9, 192–198. [Google Scholar] [CrossRef]
- Ye, W.; Liu, L.; Wang, Z.; Yu, J.; Fan, Y. Investigation of Pretreatment Methods for Improving TEMPO-Mediated Oxidation and Nanofibrillation Efficiency of α-Chitin. ACS Sustain. Chem. Eng. 2019, 7, 19463–19473. [Google Scholar] [CrossRef]
- Kiroshka, V.V.; Petrova, V.A.; Chernyakov, D.D.; Bozhkova, Y.O.; Kiroshka, K.V.; Baklagina, Y.G.; Romanov, D.P.; Kremnev, R.V.; Skorik, Y.A. Influence of Chitosan-Chitin Nanofiber Composites on Cytoskeleton Structure and the Proliferation of Rat Bone Marrow Stromal Cells. J. Mater. Sci. Mater. Med. 2017, 28, 21. [Google Scholar] [CrossRef]
- Coltelli, M.-B.; Gigante, V.; Panariello, L.; Aliotta, L.; Morganti, P.; Danti, S.; Cinelli, P.; Lazzeri, A. Chitin Nanofibrils in Renewable Materials for Packaging and Personal Care Applications. Adv. Mater. Lett. 2019, 10, 425–430. [Google Scholar] [CrossRef]
- Panariello, L.; Coltelli, M.-B.; Buchignani, M.; Lazzeri, A. Chitosan and Nano-Structured Chitin for Biobased Anti-Microbial Treatments onto Cellulose Based Materials. Eur. Polym. J. 2019, 113, 328–339. [Google Scholar] [CrossRef]
- Eichhorn, S.J.; Dufresne, A.; Aranguren, M.; Marcovich, N.E.; Capadona, J.R.; Rowan, S.J.; Weder, C.; Thielemans, W.; Roman, M.; Renneckar, S.; et al. Review: Current International Research into Cellulose Nanofibres and Nanocomposites. J. Mater. Sci. 2010, 45, 1–33. [Google Scholar] [CrossRef]
- Freire, C.S.R.; Fernandes, S.C.M.; Silvestre, A.J.D.; Pascoal Neto, C. Novel Cellulose-Based Composites Based on Nanofibrillated Plant and Bacterial Cellulose: Recent Advances at the University of Aveiro—A Review. Holzforschung 2013, 67, 603–612. [Google Scholar] [CrossRef]
- Shojaeiarani, J.; Bajwa, D.S.; Chanda, S. Cellulose Nanocrystal Based Composites: A Review. Compos. Part C Open Access 2021, 5, 100164. [Google Scholar] [CrossRef]
- Abdelhamid, H.N.; Mathew, A.P. A Review on Cellulose-Based Materials for Biomedicine. Preprints 2022. [Google Scholar] [CrossRef]
- Kim, J.; Yun, S.; Ounaies, Z. Discovery of Cellulose as a Smart Material. Macromolecules 2006, 39, 4202–4206. [Google Scholar] [CrossRef]
- Henriksson, M.; Henriksson, G.; Berglund, L.A.; Lindström, T. An Environmentally Friendly Method for Enzyme-Assisted Preparation of Microfibrillated Cellulose (MFC) Nanofibers. Eur. Polym. J. 2007, 43, 3434–3441. [Google Scholar] [CrossRef]
- Cunha, I.; Martins, J.; Bahubalindruni, P.G.; Carvalho, J.T.; Rodrigues, J.; Rubin, S.; Fortunato, E.; Martins, R.; Pereira, L. Handwritten and Sustainable Electronic Logic Circuits with Fully Printed Paper Transistors (Adv. Mater. Technol. 12/2021). Adv. Mater. Technol. 2021, 6, 2170071. [Google Scholar] [CrossRef]
- Cunha, I.; Martins, J.; Bahubalindruni, P.G.; Carvalho, J.T.; Rodrigues, J.; Rubin, S.; Fortunato, E.; Martins, R.; Pereira, L. Handwritten and Sustainable Electronic Logic Circuits with Fully Printed Paper Transistors. Adv. Mater. Technol. 2021, 6, 2100633. [Google Scholar] [CrossRef]
- Mondragon, G.; Fernandes, S.; Retegi, A.; Peña, C.; Algar, I.; Eceiza, A.; Arbelaiz, A. A Common Strategy to Extracting Cellulose Nanoentities from Different Plants. Ind. Crops Prod. 2014, 55, 140–148. [Google Scholar] [CrossRef]
- Bondeson, D.; Mathew, A.; Oksman, K. Optimization of the Isolation of Nanocrystals from Microcrystalline Cellulose by Acid Hydrolysis. Cellulose 2006, 13, 171–180. [Google Scholar] [CrossRef]
- Nissilä, T.; Wei, J.; Geng, S.; Teleman, A.; Oksman, K. Ice-Templated Cellulose Nanofiber Filaments as a Reinforcement Material in Epoxy Composites. Nanomaterials 2021, 11, 490. [Google Scholar] [CrossRef]
- Muraleedharan, M.N.; Karnaouri, A.; Piatkova, M.; Ruiz-Caldas, M.-X.; Matsakas, L.; Liu, B.; Rova, U.; Christakopoulos, P.; Mathew, A.P. Isolation and Modification of Nano-Scale Cellulose from Organosolv-Treated Birch through the Synergistic Activity of LPMO and Endoglucanases. Int. J. Biol. Macromol. 2021, 183, 101–109. [Google Scholar] [CrossRef]
- Mathew, A.P.; Oksman, K.; Sain, M. Mechanical Properties of Biodegradable Composites from Poly Lactic Acid (PLA) and Microcrystalline Cellulose (MCC). J. Appl. Polym. Sci. 2005, 97, 2014–2025. [Google Scholar] [CrossRef]
- Oksman, K.; Mathew, A.P.; Bondeson, D.; Kvien, I. Manufacturing Process of Cellulose Whiskers/Polylactic Acid Nanocomposites. Compos. Sci. Technol. 2006, 66, 2776–2784. [Google Scholar] [CrossRef]
- Jonoobi, M.; Harun, J.; Mathew, A.P.; Oksman, K. Mechanical Properties of Cellulose Nanofiber (CNF) Reinforced Polylactic Acid (PLA) Prepared by Twin Screw Extrusion. Compos. Sci. Technol. 2010, 70, 1742–1747. [Google Scholar] [CrossRef]
- Berglund, L.; Nissilä, T.; Sivaraman, D.; Komulainen, S.; Telkki, V.-V.; Oksman, K. Seaweed-Derived Alginate–Cellulose Nanofiber Aerogel for Insulation Applications. ACS Appl. Mater. Interfaces 2021, 13, 34899–34909. [Google Scholar] [CrossRef]
- Fijoł, N.; Aguilar-Sánchez, A.; Mathew, A.P. 3D-Printable Biopolymer-Based Materials for Water Treatment: A Review. Chem. Eng. J. 2022, 430, 132964. [Google Scholar] [CrossRef]
- Abdelhamid, H.N.; Mathew, A.P. In-Situ Growth of Zeolitic Imidazolate Frameworks into a Cellulosic Filter Paper for the Reduction of 4-Nitrophenol. Carbohydr. Polym. 2021, 274, 118657. [Google Scholar] [CrossRef]
- Sultan, S.; Mathew, A.P. 3D Printed Porous Cellulose Nanocomposite Hydrogel Scaffolds. J. Vis. Exp. 2019, 146, e59401. [Google Scholar] [CrossRef]
- Oprea, M.; Panaitescu, D.M. Nanocellulose Hybrids with Metal Oxides Nanoparticles for Biomedical Applications. Molecules 2020, 25, 4045. [Google Scholar] [CrossRef]
- Hietala, M.; Mathew, A.P.; Oksman, K. Bionanocomposites of Thermoplastic Starch and Cellulose Nanofibers Manufactured Using Twin-Screw Extrusion. Eur. Polym. J. 2013, 49, 950–956. [Google Scholar] [CrossRef]
- Oksman, K.; Aitomäki, Y.; Mathew, A.P.; Siqueira, G.; Zhou, Q.; Butylina, S.; Tanpichai, S.; Zhou, X.; Hooshmand, S. Review of the Recent Developments in Cellulose Nanocomposite Processing. Compos. Part A Appl. Sci. Manuf. 2016, 83, 2–18. [Google Scholar] [CrossRef]
- Abdelhamid, H.N.; Mathew, A.P. Cellulose–Metal Organic Frameworks (CelloMOFs) Hybrid Materials and Their Multifaceted Applications: A Review. Coord. Chem. Rev. 2022, 451, 214263. [Google Scholar] [CrossRef]
- Vadillo, J.; Larraza, I.; Calvo-Correas, T.; Gabilondo, N.; Derail, C.; Eceiza, A. Role of in Situ Added Cellulose Nanocrystals as Rheological Modulator of Novel Waterborne Polyurethane Urea for 3D-Printing Technology. Cellulose 2021, 28, 4729–4744. [Google Scholar] [CrossRef]
- Thomas, P.; Duolikun, T.; Rumjit, N.P.; Moosavi, S.; Lai, C.W.; Bin Johan, M.R.; Fen, L.B. Comprehensive Review on Nanocellulose: Recent Developments, Challenges and Future Prospects. J. Mech. Behav. Biomed. Mater. 2020, 110, 103884. [Google Scholar] [CrossRef]
- Zinge, C.; Kandasubramanian, B. Nanocellulose Based Biodegradable Polymers. Eur. Polym. J. 2020, 133, 109758. [Google Scholar] [CrossRef]
- Tomita, Y.; Kondo, T. Influential Factors to Enhance the Moving Rate of Acetobacter Xylinum Due to Its Nanofiber Secretion on Oriented Templates. Carbohydr. Polym. 2009, 77, 754–759. [Google Scholar] [CrossRef]
- George, J.; Ramana, K.V.; Sabapathy, S.N.; Jagannath, J.H.; Bawa, A.S. Characterization of Chemically Treated Bacterial (Acetobacter xylinum) Biopolymer: Some Thermo-Mechanical Properties. Int. J. Biol. Macromol. 2005, 37, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Esa, F.; Tasirin, S.M.; Rahman, N.A. Overview of Bacterial Cellulose Production and Application. Agric. Agric. Sci. Procedia 2014, 2, 113–119. [Google Scholar] [CrossRef]
- Wang, J.; Tavakoli, J.; Tang, Y. Bacterial Cellulose Production, Properties and Applications with Different Culture Methods—A Review. Carbohydr. Polym. 2019, 219, 63–76. [Google Scholar] [CrossRef]
- Carreira, P.; Mendes, J.A.S.; Trovatti, E.; Serafim, L.S.; Freire, C.S.R.; Silvestre, A.J.D.; Neto, C.P. Utilization of Residues from Agro-Forest Industries in the Production of High Value Bacterial Cellulose. Bioresour. Technol. 2011, 102, 7354–7360. [Google Scholar] [CrossRef] [PubMed]
- Algar, I.; Fernandes, S.C.M.; Mondragon, G.; Castro, C.; Garcia-Astrain, C.; Gabilondo, N.; Retegi, A.; Eceiza, A. Pineapple Agroindustrial Residues for the Production of High Value Bacterial Cellulose with Different Morphologies. J. Appl. Polym. Sci. 2015, 132, 41237. [Google Scholar] [CrossRef]
- Gomes, F.P.; Silva, N.H.C.S.; Trovatti, E.; Serafim, L.S.; Duarte, M.F.; Silvestre, A.J.D.; Neto, C.P.; Freire, C.S.R. Production of Bacterial Cellulose by Gluconacetobacter sacchari Using Dry Olive Mill Residue. Biomass Bioenergy 2013, 55, 205–211. [Google Scholar] [CrossRef]
- Urbina, L.; Hernández-Arriaga, A.M.; Eceiza, A.; Gabilondo, N.; Corcuera, M.A.; Prieto, M.A.; Retegi, A. By-Products of the Cider Production: An Alternative Source of Nutrients to Produce Bacterial Cellulose. Cellulose 2017, 24, 2071–2082. [Google Scholar] [CrossRef]
- Fernandes, S.C.M.; Oliveira, L.; Freire, C.S.R.; Silvestre, A.J.D.; Neto, C.P.; Gandini, A.; Desbriéres, J. Novel Transparent Nanocomposite Films Based on Chitosan and Bacterial Cellulose. Green Chem. 2009, 11, 2023. [Google Scholar] [CrossRef]
- Trovatti, E.; Fernandes, S.C.M.; Rubatat, L.; Freire, C.S.R.; Silvestre, A.J.D.; Neto, C.P. Sustainable Nanocomposite Films Based on Bacterial Cellulose and Pullulan. Cellulose 2012, 19, 729–737. [Google Scholar] [CrossRef]
- Fernandes, S.C.M.; Sadocco, P.; Alonso-Varona, A.; Palomares, T.; Eceiza, A.; Silvestre, A.J.D.; Mondragon, I.; Freire, C.S.R. Bioinspired Antimicrobial and Biocompatible Bacterial Cellulose Membranes Obtained by Surface Functionalization with Aminoalkyl Groups. ACS Appl. Mater. Interfaces 2013, 5, 3290–3297. [Google Scholar] [CrossRef]
- Vilela, C.; Silva, A.C.Q.; Domingues, E.M.; Gonçalves, G.; Martins, M.A.; Figueiredo, F.M.L.; Santos, S.A.O.; Freire, C.S.R. Conductive Polysaccharides-Based Proton-Exchange Membranes for Fuel Cell Applications: The Case of Bacterial Cellulose and Fucoidan. Carbohydr. Polym. 2020, 230, 115604. [Google Scholar] [CrossRef]
- Almeida, T.; Silvestre, A.J.D.; Vilela, C.; Freire, C.S.R. Bacterial Nanocellulose toward Green Cosmetics: Recent Progresses and Challenges. Int. J. Mol. Sci. 2021, 22, 2836. [Google Scholar] [CrossRef]
- Frazão, C.J.R.; Silva, N.H.C.; Freire, C.S.R.; Silvestre, A.J.D.; Xavier, A.M.R.B.; Tavares, A.P.M. Bacterial Cellulose as Carrier for Immobilization of Laccase: Optimization and Characterization. Eng. Life Sci. 2014, 14, 500–508. [Google Scholar] [CrossRef]
- Silva, N.H.C.S.; Rodrigues, A.F.; Almeida, I.F.; Costa, P.C.; Rosado, C.; Neto, C.P.; Silvestre, A.J.D.; Freire, C.S.R. Bacterial Cellulose Membranes as Transdermal Delivery Systems for Diclofenac: In Vitro Dissolution and Permeation Studies. Carbohydr. Polym. 2014, 106, 264–269. [Google Scholar] [CrossRef]
- Trovatti, E.; Freire, C.S.R.; Pinto, P.C.; Almeida, I.F.; Costa, P.; Silvestre, A.J.D.; Neto, C.P.; Rosado, C. Bacterial Cellulose Membranes Applied in Topical and Transdermal Delivery of Lidocaine Hydrochloride and Ibuprofen: In Vitro Diffusion Studies. Int. J. Pharm. 2012, 435, 83–87. [Google Scholar] [CrossRef]
- Trovatti, E.; Oliveira, L.; Freire, C.S.R.; Silvestre, A.J.D.; Pascoal Neto, C.; Cruz Pinto, J.J.C.; Gandini, A. Novel Bacterial Cellulose–Acrylic Resin Nanocomposites. Compos. Sci. Technol. 2010, 70, 1148–1153. [Google Scholar] [CrossRef]
- Figueiredo, A.G.P.R.; Figueiredo, A.R.P.; Alonso-Varona, A.; Fernandes, S.C.M.; Palomares, T.; Rubio-Azpeitia, E.; Barros-Timmons, A.; Silvestre, A.J.D.; Pascoal Neto, C.; Freire, C.S.R. Biocompatible Bacterial Cellulose-Poly(2-Hydroxyethyl Methacrylate) Nanocomposite Films. BioMed Res. Int. 2013, 2013, 698141. [Google Scholar] [CrossRef]
- Tomé, L.C.; Brandão, L.; Mendes, A.M.; Silvestre, A.J.D.; Neto, C.P.; Gandini, A.; Freire, C.S.R.; Marrucho, I.M. Preparation and Characterization of Bacterial Cellulose Membranes with Tailored Surface and Barrier Properties. Cellulose 2010, 17, 1203–1211. [Google Scholar] [CrossRef]
- Figueiredo, A.R.P.; Silvestre, A.J.D.; Neto, C.P.; Freire, C.S.R. In Situ Synthesis of Bacterial Cellulose/Polycaprolactone Blends for Hot Pressing Nanocomposite Films Production. Carbohydr. Polym. 2015, 132, 400–408. [Google Scholar] [CrossRef]
- Kontturi, K.S.; Lee, K.-Y.; Jones, M.P.; Sampson, W.W.; Bismarck, A.; Kontturi, E. Influence of Biological Origin on the Tensile Properties of Cellulose Nanopapers. Cellulose 2021, 28, 6619–6628. [Google Scholar] [CrossRef]
- Ricard-Blum, S. The Collagen Family. Cold Spring Harb. Perspect. Biol. 2011, 3, a004978. [Google Scholar] [CrossRef]
- Deshmukh, S.N.; Dive, A.M.; Moharil, R.; Munde, P. Enigmatic Insight into Collagen. J. Oral Maxillofac. Pathol. 2016, 20, 276–283. [Google Scholar] [CrossRef]
- Avila Rodríguez, M.I.; Rodríguez Barroso, L.G.; Sánchez, M.L. Collagen: A Review on Its Sources and Potential Cosmetic Applications. J. Cosmet. Derm. 2018, 17, 20–26. [Google Scholar] [CrossRef]
- Silvipriya, K.; Kumar, K.; Bhat, A.; Kumar, B.; John, A.; Lakshmanan, P. Collagen: Animal Sources and Biomedical Application. J. Appl. Pharm. Sci. 2015, 5, 123–127. [Google Scholar] [CrossRef]
- Sionkowska, A.; Adamiak, K.; Musiał, K.; Gadomska, M. Collagen Based Materials in Cosmetic Applications: A Review. Materials 2020, 13, 4217. [Google Scholar] [CrossRef] [PubMed]
- Coppola, D.; Oliviero, M.; Vitale, G.A.; Lauritano, C.; D’Ambra, I.; Iannace, S.; De Pascale, D. Marine Collagen from Alternative and Sustainable Sources: Extraction, Processing and Applications. Mar. Drugs 2020, 18, 214. [Google Scholar] [CrossRef] [PubMed]
- Ricard-Blum, S.; Tiollier, J.; Garrone, R.; Herbage, D. Further Biochemical and Physicochemical Characterization of Minor Disulfide-Bonded (Type IX) Collagen, Extracted from Foetal Calf Cartilage. J. Cell. Biochem. 1985, 27, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Dubost, A.; Micol, D.; Meunier, B.; Lethias, C.; Listrat, A. Relationships between Structural Characteristics of Bovine Intramuscular Connective Tissue Assessed by Image Analysis and Collagen and Proteoglycan Content. Meat Sci. 2013, 93, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Exposito, J.-Y.; Valcourt, U.; Cluzel, C.; Lethias, C. ChemInform Abstract: The Fibrillar Collagen Family. ChemInform 2011, 42, 407–426. [Google Scholar] [CrossRef]
- Margaron, Y.; Bostan, L.; Exposito, J.-Y.; Malbouyres, M.; Trunfio-Sfarghiu, A.-M.; Berthier, Y.; Lethias, C. Tenascin-X Increases the Stiffness of Collagen Gels without Affecting Fibrillogenesis. Biophys. Chem. 2010, 147, 87–91. [Google Scholar] [CrossRef]
- Lethias, C.; Carisey, A.; Comte, J.; Cluzel, C.; Exposito, J.-Y. A Model of Tenascin-X Integration within the Collagenous Network. FEBS Lett. 2006, 580, 6281–6285. [Google Scholar] [CrossRef]
- Aouacheria, A.; Cluzel, C.; Lethias, C.; Gouy, M.; Garrone, R.; Exposito, J.-Y. Invertebrate Data Predict an Early Emergence of Vertebrate Fibrillar Collagen Clades and an Anti-Incest Model. J. Biol. Chem. 2004, 279, 47711–47719. [Google Scholar] [CrossRef]
- Sionkowska, A.; Lewandowska, K.; Adamiak, K. The Influence of UV Light on Rheological Properties of Collagen Extracted from Silver Carp Skin. Materials 2020, 13, 4453. [Google Scholar] [CrossRef]
- Sionkowska, A.; Kamińska, A. Thermal Helix-Coil Transition in UV Irradiated Collagen from Rat Tail Tendon. Int. J. Biol. Macromol. 1999, 24, 337–340. [Google Scholar] [CrossRef]
- Katz, L.; Baltz, R.H. Natural Product Discovery: Past, Present, and Future. J. Ind. Microbiol. Biotechnol. 2016, 43, 155–176. [Google Scholar] [CrossRef]
- Colliec-Jouault, S.; Zanchetta, P.; Helley, D.; Ratiskol, J.; Sinquin, C.; Fischer, A.M.; Guezennec, J. Les polysaccharides microbiens d’origine marine et leur potentiel en thérapeutique humaine. Pathol. Biol. 2004, 52, 127–130. (In French) [Google Scholar] [CrossRef]
- Raposo, M.; De Morais, R.; Bernardo de Morais, A. Bioactivity and Applications of Sulphated Polysaccharides from Marine Microalgae. Mar. Drugs 2013, 11, 233–252. [Google Scholar] [CrossRef]
- Laurienzo, P. Marine Polysaccharides in Pharmaceutical Applications: An Overview. Mar. Drugs 2010, 8, 2435–2465. [Google Scholar] [CrossRef]
- Delbarre-Ladrat, C.; Salas, M.L.; Sinquin, C.; Zykwinska, A.; Colliec-Jouault, S. Bioprospecting for Exopolysaccharides from Deep-Sea Hydrothermal Vent Bacteria: Relationship between Bacterial Diversity and Chemical Diversity. Microorganisms 2017, 5, 63. [Google Scholar] [CrossRef]
- Zykwinska, A.; Marchand, L.; Bonnetot, S.; Sinquin, C.; Colliec-Jouault, S.; Delbarre-Ladrat, C. Deep-Sea Hydrothermal Vent Bacteria as a Source of Glycosaminoglycan-Mimetic Exopolysaccharides. Molecules 2019, 24, 1703. [Google Scholar] [CrossRef]
- Colliec Jouault, S.; Chevolot, L.; Helley, D.; Ratiskol, J.; Bros, A.; Sinquin, C.; Roger, O.; Fischer, A.-M. Characterization, Chemical Modifications and in Vitro Anticoagulant Properties of an Exopolysaccharide Produced by Alteromonas Infernus. Biochim. Biophys. Acta (BBA) Gen. Subj. 2001, 1528, 141–151. [Google Scholar] [CrossRef]
- Le Costaouëc, T.; Cérantola, S.; Ropartz, D.; Ratiskol, J.; Sinquin, C.; Colliec-Jouault, S.; Boisset, C. Structural Data on a Bacterial Exopolysaccharide Produced by a Deep-Sea Alteromonas Macleodii Strain. Carbohydr. Polym. 2012, 90, 49–59. [Google Scholar] [CrossRef]
- Colliec-Jouault, S.; Zykwinska, A. Marine Glycosaminoglycans (GAGs) and GAG-Mimetics: Applications in Medicine and Tissue Engineering. In Extracellular Sugar-Based Biopolymers Matrices; Cohen, E., Merzendorfer, H., Eds.; Biologically-Inspired Systems; Springer International Publishing: Cham, Switzerland, 2019; Volume 12, pp. 625–648. ISBN 978-3-030-12918-7. [Google Scholar]
- Senni, K.; Gueniche, F.; Changotade, S.; Septier, D.; Sinquin, C.; Ratiskol, J.; Lutomski, D.; Godeau, G.; Guezennec, J.; Colliec-Jouault, S. Unusual Glycosaminoglycans from a Deep Sea Hydrothermal Bacterium Improve Fibrillar Collagen Structuring and Fibroblast Activities in Engineered Connective Tissues. Mar. Drugs 2013, 11, 1351–1369. [Google Scholar] [CrossRef]
- Delbarre-Ladrat, C.; Sinquin, C.; Lebellenger, L.; Zykwinska, A.; Colliec-Jouault, S. Exopolysaccharides Produced by Marine Bacteria and Their Applications as Glycosaminoglycan-like Molecules. Front. Chem. 2014, 2, 85. [Google Scholar] [CrossRef]
- Amjres, H.; Béjar, V.; Quesada, E.; Carranza, D.; Abrini, J.; Sinquin, C.; Ratiskol, J.; Colliec-Jouault, S.; Llamas, I. Characterization of Haloglycan, an Exopolysaccharide Produced by Halomonas Stenophila HK30. Int. J. Biol. Macromol. 2015, 72, 117–124. [Google Scholar] [CrossRef]
- Mota, R.; Vidal, R.; Pandeirada, C.; Flores, C.; Adessi, A.; De Philippis, R.; Nunes, C.; Coimbra, M.A.; Tamagnini, P. Cyanoflan: A Cyanobacterial Sulfated Carbohydrate Polymer with Emulsifying Properties. Carbohydr. Polym. 2020, 229, 115525. [Google Scholar] [CrossRef]
- Costa, R.; Costa, L.; Rodrigues, I.; Meireles, C.; Soares, R.; Tamagnini, P.; Mota, R. Biocompatibility of the Biopolymer Cyanoflan for Applications in Skin Wound Healing. Mar. Drugs 2021, 19, 147. [Google Scholar] [CrossRef]
- Nichols, C.M.; Bowman, J.P.; Guezennec, J. Olleya marilimosa gen. nov., sp. nov., an Exopolysaccharide-Producing Marine Bacterium from the Family Flavobacteriaceae, Isolated from the Southern Ocean. Int. J. Syst. Evol. Microbiol. 2005, 55, 1557–1561. [Google Scholar] [CrossRef]
- Nichols, C.M.; Lardière, S.G.; Bowman, J.P.; Nichols, P.D.; Gibson, J.A.E.; Guézennec, J. Chemical Characterization of Exopolysaccharides from Antarctic Marine Bacteria. Microb. Ecol. 2005, 49, 578–589. [Google Scholar] [CrossRef]
- Mancuso Nichols, C.A.; Nairn, K.M.; Glattauer, V.; Blackburn, S.I.; Ramshaw, J.A.M.; Graham, L.D. Screening Microalgal Cultures in Search of Microbial Exopolysaccharides with Potential as Adhesives. J. Adhes. 2009, 85, 97–125. [Google Scholar] [CrossRef]
- Salmeán, A.A.; Guillouzo, A.; Duffieux, D.; Jam, M.; Matard-Mann, M.; Larocque, R.; Pedersen, H.L.; Michel, G.; Czjzek, M.; Willats, W.G.T.; et al. Double Blind Microarray-Based Polysaccharide Profiling Enables Parallel Identification of Uncharacterized Polysaccharides and Carbohydrate-Binding Proteins with Unknown Specificities. Sci. Rep. 2018, 8, 2500. [Google Scholar] [CrossRef]
- Chernysheva, N.; Bystritskaya, E.; Stenkova, A.; Golovkin, I.; Nedashkovskaya, O.; Isaeva, M. Comparative Genomics and CAZyme Genome Repertoires of Marine Zobellia Amurskyensis KMM 3526T and Zobellia Laminariae KMM 3676T. Mar. Drugs 2019, 17, 661. [Google Scholar] [CrossRef]
- Vidal-Melgosa, S.; Pedersen, H.L.; Schückel, J.; Arnal, G.; Dumon, C.; Amby, D.B.; Monrad, R.N.; Westereng, B.; Willats, W.G.T. A New Versatile Microarray-Based Method for High Throughput Screening of Carbohydrate-Active Enzymes. J. Biol. Chem. 2015, 290, 9020–9036. [Google Scholar] [CrossRef]
- De Jesus Raposo, M.; De Morais, A.; De Morais, R. Emergent Sources of Prebiotics: Seaweeds and Microalgae. Mar. Drugs 2016, 14, 27. [Google Scholar] [CrossRef]
- Jia, S.; Lu, Z.; Gao, Z.; An, J.; Wu, X.; Li, X.; Dai, X.; Zheng, Q.; Sun, Y. Chitosan Oligosaccharides Alleviate Cognitive Deficits in an Amyloid-Β1–42-Induced Rat Model of Alzheimer’s Disease. Int. J. Biol. Macromol. 2016, 83, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Song, J.Y.; Alnaeeli, M.; Park, J.K. Efficient Digestion of Chitosan Using Chitosanase Immobilized on Silica-Gel for the Production of Multisize Chitooligosaccharides. Process Biochem. 2014, 49, 2107–2113. [Google Scholar] [CrossRef]
- Matard-Mann, M.; Bernard, T.; Leroux, C.; Barbeyron, T.; Larocque, R.; Préchoux, A.; Jeudy, A.; Jam, M.; Nyvall Collén, P.; Michel, G.; et al. Structural Insights into Marine Carbohydrate Degradation by Family GH16 κ-Carrageenases. J. Biol. Chem. 2017, 292, 19919–19934. [Google Scholar] [CrossRef] [PubMed]
- Naretto, A.; Fanuel, M.; Ropartz, D.; Rogniaux, H.; Larocque, R.; Czjzek, M.; Tellier, C.; Michel, G. The Agar-Specific Hydrolase ZgAgaC from the Marine Bacterium Zobellia Galactanivorans Defines a New GH16 Protein Subfamily. J. Biol. Chem. 2019, 294, 6923–6939. [Google Scholar] [CrossRef] [PubMed]




| Biopolymers | Origin and Extraction Methods | References |
|---|---|---|
| Agar | From red seaweeds of the order Gelidiales and Gracilariales | |
| Extraction methods: | ||
| [20] | |
| [21,22] | |
| [24,25,26] | |
| (Nano)Chitin | From the exoskeleton of crustaceans and arthropods, and from mollusks | |
| Chitin extraction methods: | ||
| [43,45,46,47] | |
| [48,49,50] | |
| Nanochitin extraction methods: | ||
| [74,75,76] | |
| (Nano)Cellulose | From plants and wood | |
| Cellulose extraction methods: | ||
| [85] | |
| Nanocellulose extraction methods: | ||
| [88,89,90,91,94] | |
| Bacterial Cellulose | From aerobic bacteria—genus Komagataeibacter | |
| [110,111,112,113] | |
| Collagen | From animal cartilage, fish skin, jellyfish | |
| Extraction methods: | ||
| [130,132,133] | |
| Biopolymers | Applications | References |
| Agar |
| [28] |
| [29,30,31,32,33] | |
| [35,36] | |
| (Nano)Chitin |
| [59] |
| [60] | |
| [61] | |
| [77] | |
| [78] | |
| (Nano)Cellulose |
| [86,87] |
| [90,91,92,93,94,100] | |
| [95] | |
| [95] | |
| Bacterial Cellulose |
| [114,115,116,117] |
| [119,120,121,122,123] | |
| [123,125] | |
| Collagen |
| [131] |
| [136] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veettil, U.T.; Olza, S.; Brugerolle de Fraissinette, N.; Bascans, E.; Castejón, N.; Adrien, A.; Fernández-Marín, R.; Nardin, C.; Fernandes, S.C.M. Contributions of Women in Recent Research on Biopolymer Science. Polymers 2022, 14, 1420. https://doi.org/10.3390/polym14071420
Veettil UT, Olza S, Brugerolle de Fraissinette N, Bascans E, Castejón N, Adrien A, Fernández-Marín R, Nardin C, Fernandes SCM. Contributions of Women in Recent Research on Biopolymer Science. Polymers. 2022; 14(7):1420. https://doi.org/10.3390/polym14071420
Chicago/Turabian StyleVeettil, Unnimaya Thalakkale, Sheila Olza, Nelly Brugerolle de Fraissinette, Elodie Bascans, Natalia Castejón, Amandine Adrien, Rut Fernández-Marín, Corinne Nardin, and Susana C. M. Fernandes. 2022. "Contributions of Women in Recent Research on Biopolymer Science" Polymers 14, no. 7: 1420. https://doi.org/10.3390/polym14071420