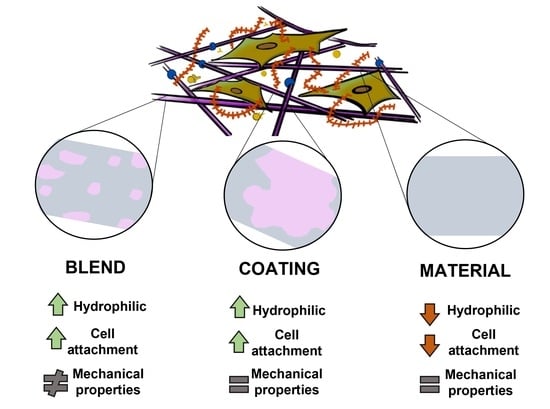
Gelatin Blends Enhance Performance of Electrospun Polymeric Scaffolds in Comparison to Coating Protocols
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Fabrication of Nanofiber Scaffolds
2.3. Characterization of Nanofibrous Scaffold
2.4. Cell Culture
2.5. Cell Viability Assay
2.6. Cell Proliferation Study on Nanofibrous Scaffolds
2.7. Statistical Analysis
3. Results and Discussion
3.1. Morphology and Mechanical Properties of Nanofibrous Scaffolds
3.2. Fourier Transform Infrared (FT-IR) Spectroscopy
3.3. Cell Proliferation Studies
3.4. MEF Adhesion and Morphology Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Park, J.S. Electrospinning and its applications. Adv. Nat. Sci. Nanosci. Nanotechnol. 2010, 1, 043002. [Google Scholar] [CrossRef] [Green Version]
- Sadasivuni, K.K.; Ponnamma, D.; Rajan, M.; Ahmed, B.; Al-Maadeed, M.A.S. (Eds.) Polymer Nanocomposites in Biomedical Engineering; Springer: Berlin/Heidelberg, Germany, 2019; ISBN 9783030047405. [Google Scholar]
- Beachley, V.; Wen, X. Effect of electrospinning parameters on the nanofiber diameter and length. Mater. Sci. Eng. C 2009, 29, 663–668. [Google Scholar] [CrossRef] [Green Version]
- Nair, L.S.; Laurencin, C.T. Biodegradable polymers as biomaterials. Prog. Polym. Sci. 2007, 32, 762–798. [Google Scholar] [CrossRef]
- Prabhakaran, M.P.; Venugopal, J.; Ramakrishna, S. Electrospun nanostructured scaffolds for bone tissue engineering. Acta Biomater. 2009, 5, 2884–2893. [Google Scholar] [CrossRef] [PubMed]
- Muniyandi, P.; Palaninathan, V.; Veeranarayanan, S.; Ukai, T.; Maekawa, T.; Hanajiri, T.; Mohamed, M.S. ECM mimetic electrospun porous poly (l-lactic acid) (PLLA) scaffolds as potential substrates for cardiac tissue engineering. Polymers 2020, 12, 451. [Google Scholar] [CrossRef] [Green Version]
- Kumbar, S.G.; Nukavarapu, S.P.; James, R.; Nair, L.S.; Laurencin, C.T. Electrospun poly(lactic acid-co-glycolic acid) scaffolds for skin tissue engineering. Biomaterials 2008, 29, 4100–4107. [Google Scholar] [CrossRef] [Green Version]
- Basu, P.; Repanas, A.; Chatterjee, A.; Glasmacher, B.; NarendraKumar, U.; Manjubala, I. PEO–CMC blend nanofibers fabrication by electrospinning for soft tissue engineering applications. Mater. Lett. 2017, 195, 10–13. [Google Scholar] [CrossRef]
- Alavarse, A.C.; de Oliveira Silva, F.W.; Colque, J.T.; da Silva, V.M.; Prieto, T.; Venancio, E.C.; Bonvent, J.J. Tetracycline hydrochloride-loaded electrospun nanofibers mats based on PVA and chitosan for wound dressing. Mater. Sci. Eng. C 2017, 77, 271–281. [Google Scholar] [CrossRef]
- Bliley, J.M.; Marra, K.G. Polymeric Biomaterials as Tissue Scaffolds; Elsevier Inc.: Amsterdam, The Netherlands, 2015; ISBN 9780123977786. [Google Scholar]
- Eshraghi, S.; Das, S. Mechanical and microstructural properties of polycaprolactone scaffolds with one-dimensional, two-dimensional, and three-dimensional orthogonally oriented porous architectures produced by selective laser sintering. Acta Biomater. 2010, 6, 2467–2476. [Google Scholar] [CrossRef] [Green Version]
- López-Rodríguez, N.; López-Arraiza, A.; Meaurio, E.; Sarasua, J.R. Crystallization, morphology, and mechanical behavior of polylactide/poly(ε-caprolactone) blends. Polym. Eng. Sci. 2006, 46, 1299–1308. [Google Scholar] [CrossRef]
- Gunatillake, P.A.; Adhikari, R.; Gadegaard, N. Biodegradable synthetic polymers for tissue engineering. Eur. Cells Mater. 2003, 5, 1–16. [Google Scholar] [CrossRef]
- Yao, R.; He, J.; Meng, G.; Jiang, B.; Wu, F. Electrospun PCL/Gelatin composite fibrous scaffolds: Mechanical properties and cellular responses. J. Biomater. Sci. Polym. Ed. 2016, 27, 824–838. [Google Scholar] [CrossRef] [PubMed]
- Abedalwafa, M.; Wang, F.; Wang, L.; Li, C. Biodegradable PCL for tissue engineering applications: A review. Rev. Adv. Mater. Sci. 2013, 34, 123–140. [Google Scholar]
- Ghasemi-Mobarakeh, L.; Prabhakaran, M.P.; Morshed, M.; Nasr-Esfahani, M.H.; Ramakrishna, S. Electrospun poly(ε-caprolactone)/gelatin nanofibrous scaffolds for nerve tissue engineering. Biomaterials 2008, 29, 4532–4539. [Google Scholar] [CrossRef] [PubMed]
- Rahmati, M.; Mills, D.K.; Urbanska, A.M.; Saeb, M.R.; Venugopal, J.R.; Ramakrishna, S.; Mozafari, M. Electrospinning for tissue engineering applications. Prog. Mater. Sci. 2021, 117, 100721. [Google Scholar] [CrossRef]
- Khorshidi, S.; Solouk, A.; Mirzadeh, H.; Mazinani, S.; Lagaron, J.M.; Sharifi, S.; Ramakrishna, S. A review o key challenges of electrospun scaffolds for tissue engineering applications. J. Tissue Eng. Regen. Med. 2014, 12, 181–204. [Google Scholar] [CrossRef]
- Cheng, Q.; Lee, B.L.P.; Komvopoulos, K.; Yan, Z.; Li, S. Plasma surface chemical treatment of electrospun poly(L-lactide) microfibrous scaffolds for enhanced cell adhesion, growth, and infiltration. Tissue Eng.-Part A 2013, 19, 1188–1198. [Google Scholar] [CrossRef] [Green Version]
- Mashhadikhan, M.; Soleimani, M.; Parivar, K.; Yaghmaei, P. ADSCs on PLLA/PCL hybrid nanoscaffold and gelatin modification: Cytocompatibility and mechanical properties. Avicenna J. Med. Biotechnol. 2015, 7, 32–38. [Google Scholar]
- Yang, X.; Li, Y.; He, W.; Huang, Q.; Zhang, R.; Feng, Q. Hydroxyapatite/collagen coating on PLGA electrospun fibers for osteogenic differentiation of bone marrow mesenchymal stem cells. J. Biomed. Mater. Res.-Part A 2018, 106, 2863–2870. [Google Scholar] [CrossRef]
- Biazar, E.; Keshel, S.H. Gelatin-modified nanofibrous PHBV tube as artificial nerve graft for rat sciatic nerve regeneration. Int. J. Polym. Mater. Polym. Biomater. 2014, 63, 330–336. [Google Scholar] [CrossRef]
- Ren, K.; Wang, Y.; Sun, T.; Yue, W.; Zhang, H. Electrospun PCL/gelatin composite nanofiber structures for effective guided bone regeneration membranes. Mater. Sci. Eng. C 2017, 78, 324–332. [Google Scholar] [CrossRef]
- Schiffman, J.D.; Schauer, C.L. A review: Electrospinning of biopolymer nanofibers and their applications. Polym. Rev. 2008, 48, 317–352. [Google Scholar] [CrossRef]
- Aldana, A.A.; Abraham, G.A. Current advances in electrospun gelatin-based scaffolds for tissue engineering applications. Int. J. Pharm. 2017, 523, 441–453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Croisier, F.; Duwez, A.S.; Jérôme, C.; Léonard, A.F.; Van Der Werf, K.O.; Dijkstra, P.J.; Bennink, M.L. Mechanical testing of electrospun PCL fibers. Acta Biomater. 2012, 8, 218–224. [Google Scholar] [CrossRef]
- Huang, Z.M.; Zhang, Y.Z.; Ramakrishna, S.; Lim, C.T. Electrospinning and mechanical characterization of gelatin nanofibers. Polymer 2004, 45, 5361–5368. [Google Scholar] [CrossRef]
- Nagam Hanumantharao, S.; Rao, S. Multi-Functional Electrospun Nanofibers from polymer blends for scaffold tissue engineering. Fibers 2019, 7, 66. [Google Scholar]
- Heidari, M.; Bahrami, S.H.; Ranjbar-Mohammadi, M.; Milan, P.B. Smart electrospun nanofibers containing PCL/gelatin/graphene oxide for application in nerve tissue engineering. Mater. Sci. Eng. C 2019, 103, 109768. [Google Scholar] [CrossRef]
- Xue, J.; He, M.; Liu, H.; Niu, Y.; Crawford, A.; Coates, P.D.; Chen, D.; Shi, R.; Zhang, L. Drug loaded homogeneous electrospun PCL/gelatin hybrid nanofiber structures for anti-infective tissue regeneration membranes. Biomaterials 2014, 35, 9395–9405. [Google Scholar] [CrossRef] [Green Version]
- Rahmani, S.; Tabandeh, F.; Faghihi, S.; Amoabediny, G.; Shakibaie, M.; Noorani, B.; Yazdian, F. Fabrication and characterization of poly(ε-caprolactone)/gelatin nanofibrous scaffolds for retinal tissue engineering. Int. J. Polym. Mater. Polym. Biomater. 2018, 67, 27–35. [Google Scholar] [CrossRef]
- Jafari, A.; Amirsadeghi, A.; Hassanajili, S.; Azarpira, N. Bioactive antibacterial bilayer PCL/gelatin nanofibrous scaffold promotes full-thickness wound healing. Int. J. Pharm. 2020, 583, 119413. [Google Scholar] [CrossRef]
- Rather, H.A.; Thakore, R.; Singh, R.; Jhala, D.; Singh, S.; Vasita, R. Antioxidative study of Cerium Oxide nanoparticle functionalised PCL-Gelatin electrospun fibers for wound healing application. Bioact. Mater. 2018, 3, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Gautam, S.; Dinda, A.K.; Mishra, N.C. Fabrication and characterization of PCL/gelatin composite nanofibrous scaffold for tissue engineering applications by electrospinning method. Mater. Sci. Eng. C 2013, 33, 1228–1235. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, G.; Cai, Y.; Monkley, S.J.; Critchley, D.R.; Sheetz, M.P. Talin depletion reveals independence of initial cell spreading from integrin activation and traction. Nat. Cell Biol. 2008, 10, 1062–1068. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roca-cusachs, P.; Puklin-faucher, E.; Gauthier, N.C.; Biais, N. Integrin-dependent force transmission to the extracellular matrix by α-actinin triggers adhesion maturation. Proc. Nat. Acad. Sci. USA 2013, 110, E1361–E1370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Safaeijavan, R.; Soleimani, M.; Divsalar, A.; Eidi, A.; Ardeshirylajimi, A. Biological behaviour study of gelatin coated PCL nanofibrous electrospun scaffolds using fibroblasts. Arch. Adv. Biosci. 2014, 5, 67–73. [Google Scholar]
- Nagiah, N.; Madhavi, L.; Anitha, R.; Anandan, C.; Srinivasan, N.T.; Sivagnanam, U.T. Development and characterization of coaxially electrospun gelatin coated poly (3-hydroxybutyric acid) thin films as potential scaffolds for skin regeneration. Mater. Sci. Eng. C 2013, 33, 4444–4452. [Google Scholar] [CrossRef]
- Suresh, S.; Gryshkov, O.; Glasmacher, B. Impact of setup orientation on blend electrospinning of poly-ε-caprolactonegelatin scaffolds for vascular tissue engineering. Int. J. Artif. Organs 2018, 41, 801–810. [Google Scholar] [CrossRef]
- Lim, Y.C.; Johnson, J.; Fei, Z.; Wu, Y.; Farson, D.F.; Lannutti, J.J.; Choi, H.W.; Lee, L.J. Micropatterning and characterization of electrospun poly(ε-caprolactone)/gelatin nanofiber tissue scaffolds by femtosecond laser ablation for tissue engineering applications. Biotechnol. Bioeng. 2011, 108, 116–126. [Google Scholar] [CrossRef]
- Lee, J.; Tae, G.; Kim, Y.H.; Park, I.S.; Kim, S.H.; Kim, S.H. The effect of gelatin incorporation into electrospun poly(l-lactide-co-ε-caprolactone) fibers on mechanical properties and cytocompatibility. Biomaterials 2008, 29, 1872–1879. [Google Scholar] [CrossRef]
- Wu, S.C.; Chang, W.H.; Dong, G.C.; Chen, K.Y.; Chen, Y.S.; Yao, C.H. Cell adhesion and proliferation enhancement by gelatin nanofiber scaffolds. J. Bioact. Compat. Polym. 2011, 26, 565–577. [Google Scholar] [CrossRef]
- Su, Y.; Li, X.; Liu, S.; Wang, H.; He, C. Fabrication and properties of PLLA—Gelatin nanofibers by electropsinning. J. Appl. Polym. Sci. 2010, 116, 2658–2667. [Google Scholar]
- Mo, X.M.; Xu, C.Y.; Kotaki, M.; Ramakrishna, S. Electrospun P(LLA-CL) nanofiber: A biomimetic extracellular matrix for smooth muscle cell and endothelial cell proliferation. Biomaterials 2004, 25, 1883–1890. [Google Scholar] [CrossRef] [PubMed]
- Charrier, E.E.; Pogoda, K.; Wells, R.G.; Janmey, P.A. Control of cell morphology and differentiation by substrates with independently tunable elasticity and viscous dissipation. Nat. Commun. 2018, 9, 449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McBeath, R.; Pirone, D.; Belson, C. Celll shape, cytoskeletal tension, and RhoA regulate stem cell linage commitment. Dev. Cell 2004, 6, 483–495. [Google Scholar]
- Nava, M.; Raimondi, M.; Pietrabissa, R. Controlling self-renewal and differentiation of stem cells via mechanical cues. BioMed Res. Int. 2012, 2012, 797410. [Google Scholar]
- Yi, B.; Shen, Y.; Tang, H.; Wang, X.; Li, B.; Zhang, Y. Stiffness of Aligned Fibers Regulates the Phenotypic Expression of Vascular Smooth Muscle Cells. ACS Appl. Mater. Interfaces 2019, 11, 6867–6880. [Google Scholar] [CrossRef]
- Kishan, A.P.; Cosgriff-Hernandez, E.M. Recent advancements in electrospinning design for tissue engineering applications: A review. J. Biomed. Mater. Res. Part A 2017, 105, 2892–2905. [Google Scholar] [CrossRef]
- Samavedi, S.; Olsen Horton, C.; Guelcher, S.A.; Goldstein, A.S.; Whittington, A.R. Fabrication of a model continuously graded co-electrospun mesh for regeneration of the ligament-bone interface. Acta Biomater. 2011, 7, 4131–4138. [Google Scholar] [CrossRef]
- Detta, N.; Errico, C.; Dinucci, D.; Puppi, D.; Clarke, D.A.; Reilly, G.C.; Chiellini, F. Novel electrospun polyurethane/gelatin composite meshes for vascular grafts. J. Mater. Sci. Mater. Med. 2010, 21, 1761–1769. [Google Scholar] [CrossRef]
- D’Angelo, M.; Benedetti, E.; Tupone, M.G.; Catanesi, M.; Castelli, V.; Antonosante, A.; Cimini, A. The Role of Stiffness in Cell Reprogramming: A Potential Role for Biomaterials in Inducing Tissue Regeneration. Cells 2019, 8, 1036. [Google Scholar] [CrossRef] [Green Version]
- Chaudhuri, O.; Cooper-White, J.; Janmey, P.A.; Mooney, D.J.; Shenoy, V.B. Effects of extracellular matrix viscoelasticity on cellular behaviour. Nature 2020, 584, 535–546. [Google Scholar] [CrossRef] [PubMed]







| Substrate | Thickness (μm) | Fiber Diameter (nm) | Pore Size (μm2) |
|---|---|---|---|
| PCL | 20.25 ± 1.25 | 702 ± 268 | 2.1 ± 3.5 |
| PCL coated with gelatin | 20.75 ±1.70 | 804 ± 150 | 0.9 ± 2.9 |
| PCL/gelatin (80:20) | 19.65 ± 7.68 | 337 ± 106 | 1.2 ± 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bikuna-Izagirre, M.; Aldazabal, J.; Paredes, J. Gelatin Blends Enhance Performance of Electrospun Polymeric Scaffolds in Comparison to Coating Protocols. Polymers 2022, 14, 1311. https://doi.org/10.3390/polym14071311
Bikuna-Izagirre M, Aldazabal J, Paredes J. Gelatin Blends Enhance Performance of Electrospun Polymeric Scaffolds in Comparison to Coating Protocols. Polymers. 2022; 14(7):1311. https://doi.org/10.3390/polym14071311
Chicago/Turabian StyleBikuna-Izagirre, Maria, Javier Aldazabal, and Jacobo Paredes. 2022. "Gelatin Blends Enhance Performance of Electrospun Polymeric Scaffolds in Comparison to Coating Protocols" Polymers 14, no. 7: 1311. https://doi.org/10.3390/polym14071311