Novel Biodegradable Poly (Lactic Acid)/Wood Leachate Composites: Investigation of Antibacterial, Mechanical, Morphological, and Thermal Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
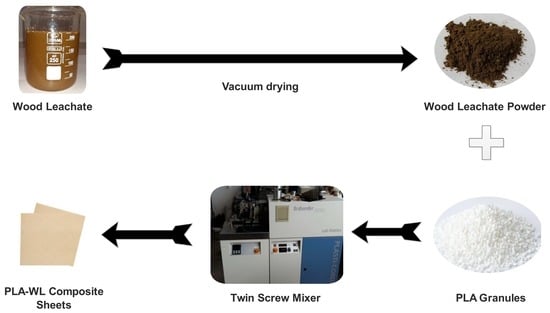
2.2. Biocomposite Preparation
2.3. Characterization
2.3.1. Structural and Thermal Analysis
2.3.2. Mechanical and Morphological Investigation
2.3.3. Surface Hydrophobicity and Antibacterial Activity
3. Results and Discussion
3.1. Structural Characterization
3.2. Mechanical Properties
3.3. Thermal Behavior
3.4. Morphology
3.5. Contact Angle
3.6. Antibacterial Behavior
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vitolina, S.; Shulga, G.; Neiberte, B.; Jaunslavietis, J.; Verovkins, A.; Betkers, T. Characteristics of the Waste Wood Biomass and Its Effect on the Properties of Wood Sanding Dust/Recycled PP Composite. Polymers 2022, 14, 468. [Google Scholar] [CrossRef] [PubMed]
- Antov, P.; Krišt’ák, L.; Réh, R.; Savov, V.; Papadopoulos, A.N. Eco-friendly fiberboard panels from recycled fibers bonded with calcium lignosulfonate. Polymers 2021, 13, 639. [Google Scholar] [CrossRef] [PubMed]
- Scaffaro, R.; Maio, A.; Sutera, F.; Gulino, E.F.; Morreale, M. Degradation and recycling of films based on biodegradable polymers: A short review. Polymers 2019, 11, 651. [Google Scholar] [CrossRef] [Green Version]
- Silva, A.L.P. Future-proofing plastic waste management for a circular bioeconomy. Curr. Opin. Environ. Sci. Health 2021, 22, 100263. [Google Scholar] [CrossRef]
- Dorieh, A.; Selakjani, P.P.; Shahavi, M.H.; Pizzi, A.; Movahed, S.G.; Pour, M.F.; Aghaei, R. Recent developments in the performance of micro/nanoparticle-modified urea-formaldehyde resins used as wood-based composite binders: A review. Int. J. Adhes. Adhes. 2022, 114, 103106. [Google Scholar] [CrossRef]
- Fonseca-García, A.; Jiménez-Regalado, E.J.; Aguirre-Loredo, R.Y. Preparation of a novel biodegradable packaging film based on corn starch-chitosan and poloxamers. Carbohydr. Polym. 2021, 251, 117009. [Google Scholar] [CrossRef]
- Ramesh, M.; Rajeshkumar, L.; Sasikala, G.; Balaji, D.; Saravanakumar, A.; Bhuvaneswari, V.; Bhoopathi, R. A Critical Review on Wood-Based Polymer Composites: Processing, Properties, and Prospects. Polymers 2022, 14, 589. [Google Scholar] [CrossRef]
- Dorieh, A.; Farajollah Pour, M.; Ghafari Movahed, S.; Pizzi, A.; Pouresmaeel Selakjani, P.; Valizadeh Kiamahalleh, M.; Hatefnia, H.; Shahavi, M.H.; Aghaei, R. A review of recent progress in melamine-formaldehyde resin based nanocomposites as coating materials. Prog. Org. Coat. 2022, 165, 106768. [Google Scholar] [CrossRef]
- Selakjani, P.P.; Dorieh, A.; Pizzi, A.; Shahavi, M.H.; Hasankhah, A.; Shekarsaraee, S.; Ashouri, M.; Movahed, S.G.; Abatari, M.N. Reducing free formaldehyde emission, improvement of thickness swelling and increasing storage stability of novel medium density fiberboard by urea-formaldehyde adhesive modified by phenol derivatives. Int. J. Adhes. Adhes. 2021, 111, 102962. [Google Scholar] [CrossRef]
- Mundel, R.; Thakur, T.; Chatterjee, M. Emerging uses of PLA–PEG copolymer in cancer drug delivery. 3 Biotech 2022, 12, 41. [Google Scholar] [CrossRef]
- Vlachopoulos, A.; Karlioti, G.; Balla, E.; Daniilidis, V.; Kalamas, T.; Stefanidou, M.; Bikiaris, N.D.; Christodoulou, E.; Koumentakou, I.; Karavas, E. Poly (Lactic Acid)-Based Microparticles for Drug Delivery Applications: An Overview of Recent Advances. Pharmaceutics 2022, 14, 359. [Google Scholar] [CrossRef]
- Abifarin, J.K.; Prakash, C.; Singh, S. Optimization and significance of fabrication parameters on the mechanical properties of 3D printed chitosan/PLA scaffold. Mater. Today Proc. 2021, 50, 2018–2025. [Google Scholar] [CrossRef]
- Młotek, M.; Gadomska-Gajadhur, A.; Sobczak, A.; Kruk, A.; Perron, M.; Krawczyk, K. Modification of PLA scaffold surface for medical applications. Appl. Sci. 2021, 11, 1815. [Google Scholar] [CrossRef]
- Fan, T.; Daniels, R. Preparation and characterization of electrospun polylactic acid (PLA) fiber loaded with birch bark triterpene extract for wound dressing. Aaps Pharmscitech 2021, 22, 1–9. [Google Scholar] [CrossRef]
- Liu, J.; Shi, R.; Hua, Y.; Gao, J.; Chen, Q.; Xu, L. A new cyanoacrylate-poly (lactic acid)-based system for a wound dressing with on-demand removal. Mater. Lett. 2021, 293, 129666. [Google Scholar] [CrossRef]
- Khosravi, A.; Fereidoon, A.; Khorasani, M.M.; Naderi, G.; Ganjali, M.R.; Zarrintaj, P.; Saeb, M.R.; Gutiérrez, T.J. Soft and hard sections from cellulose-reinforced poly (lactic acid)-based food packaging films: A critical review. Food Packag. Shelf Life 2020, 23, 100429. [Google Scholar] [CrossRef]
- Backes, E.H.; Pires, L.D.N.; Beatrice, C.A.G.; Costa, L.C.; Passador, F.R.; Pessan, L.A. Fabrication of biocompatible composites of poly (lactic acid)/hydroxyapatite envisioning medical applications. Polym. Eng. Sci. 2020, 60, 636–644. [Google Scholar] [CrossRef]
- Sin, L.T.; Rahmat, A.R.; Rahman, W.A.W.A. 3—Applications of Poly(lactic Acid). In Handbook of Biopolymers and Biodegradable Plastics, Ebnesajjad, S., Ed.; William Andrew Publishing: Boston, MA, USA, 2013; pp. 55–69. [Google Scholar]
- Alexandre, A.; Cruz Sanchez, F.A.; Boudaoud, H.; Camargo, M.; Pearce, J.M. Mechanical properties of direct waste printing of polylactic acid with universal pellets extruder: Comparison to fused filament fabrication on open-source desktop three-dimensional printers. 3D Print. Addit. Manuf. 2020, 7, 237–247. [Google Scholar] [CrossRef]
- Giorcelli, M.; Khan, A.; Pugno, N.M.; Rosso, C.; Tagliaferro, A. Biochar as a cheap and environmental friendly filler able to improve polymer mechanical properties. Biomass Bioenergy 2019, 120, 219–223. [Google Scholar] [CrossRef] [Green Version]
- Arrigo, R.; Bartoli, M.; Malucelli, G. Poly (lactic acid)–biochar biocomposites: Effect of processing and filler content on rheological, thermal, and mechanical properties. Polymers 2020, 12, 892. [Google Scholar] [CrossRef] [Green Version]
- Ebrahimpour, M.; Shahavi, M.H.; Jahanshahi, M.; Najafpour, G. Nanotechnology in Process Biotechnology: Recovery and Purification of Nanoparticulate Bioproducts Using Expanded Bed Adsorption. Dyn. Biochem. Process Biotechnol. Mol. Biol. 2009, 3, 57–60. [Google Scholar]
- Kuang, T.; Ju, J.; Liu, T.; Hejna, A.; Saeb, M.R.; Zhang, S.; Peng, X. A facile structural manipulation strategy to prepare ultra-strong, super-tough, and thermally stable polylactide/nucleating agent composites. Adv. Compos. Hybrid Mater. 2022. [Google Scholar] [CrossRef]
- Barczewski, M.; Hejna, A.; Aniśko, J.; Andrzejewski, J.; Piasecki, A.; Mysiukiewicz, O.; Bąk, M.; Gapiński, B.; Ortega, Z. Rotational molding of polylactide (PLA) composites filled with copper slag as a waste filler from metallurgical industry. Polym. Test. 2022, 106, 107449. [Google Scholar] [CrossRef]
- Scaffaro, R.; Maio, A.; Gulino, E.F.; Pitarresi, G. Lignocellulosic fillers and graphene nanoplatelets as hybrid reinforcement for polylactic acid: Effect on mechanical properties and degradability. Compos. Sci. Technol. 2020, 190, 108008. [Google Scholar] [CrossRef]
- Wolski, K.; Cichosz, S.; Masek, A. Surface hydrophobisation of lignocellulosic waste for the preparation of biothermoelastoplastic composites. Eur. Polym. J. 2019, 118, 481–491. [Google Scholar] [CrossRef]
- Singh, T.; Lendvai, L.; Dogossy, G.; Fekete, G. Physical, mechanical, and thermal properties of Dalbergia sissoo wood waste-filled poly (lactic acid) composites. Polym. Compos. 2021, 42, 4380–4389. [Google Scholar] [CrossRef]
- Moreno-Anguiano, O.; Cloutier, A.; Rutiaga-Quiñones, J.G.; Wehenkel, C.; Rosales-Serna, R.; Rebolledo, P.; Hernández-Pacheco, C.E.; Carrillo-Parra, A. Use of Agave durangensis Bagasse Fibers in the Production of Wood-Based Medium Density Fiberboard (MDF). Forests 2022, 13, 271. [Google Scholar] [CrossRef]
- Scussel, R.; Feltrin, A.C.; Angioletto, E.; Galvani, N.C.; Fagundes, M.Í.; Bernardin, A.M.; Feuser, P.E.; de Ávila, R.A.M.; Pich, C.T. Ecotoxic, genotoxic, and cytotoxic potential of leachate obtained from chromated copper arsenate-treated wood ashes. Environ. Sci. Pollut. Res. 2022, 1–14. [Google Scholar] [CrossRef]
- Raclavská, H.; Růžičková, J.; Juchelková, D.; Šafář, M.; Brťková, H.; Slamová, K. The quality of composts prepared in automatic composters from fruit waste generated by the production of beverages. Bioresour. Technol. 2021, 341, 125878. [Google Scholar] [CrossRef]
- Kannepalli, S.; Strom, P.F.; Krogmann, U.; Subroy, V.; Giménez, D.; Miskewitz, R. Characterization of wood mulch and leachate/runoff from three wood recycling facilities. J. Environ. Manag. 2016, 182, 421–428. [Google Scholar] [CrossRef]
- Zhu, Y.; Romain, C.; Williams, C.K. Sustainable polymers from renewable resources. Nature 2016, 540, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Shahavi, M.H.; Esfilar, R.; Golestani, B.; Sadeghabad, M.S.; Biglaryan, M. Comparative study of seven agricultural wastes for renewable heat and power generation using integrated gasification combined cycle based on energy and exergy analyses. Fuel 2022, 317, 123430. [Google Scholar] [CrossRef]
- Kazemeini, H.; Azizian, A.; Shahavi, M.H. Effect of Chitosan Nano-Gel/Emulsion Containing Bunium Persicum Essential Oil and Nisin as an Edible Biodegradable Coating on Escherichia Coli O157:H7 in Rainbow Trout Fillet. J. Water Environ. Nanotechnol. 2019, 4, 343–349. [Google Scholar] [CrossRef]
- Maresca, A.; Krüger, O.; Herzel, H.; Adam, C.; Kalbe, U.; Astrup, T.F. Influence of wood ash pre-treatment on leaching behaviour, liming and fertilising potential. Waste Manag. 2019, 83, 113–122. [Google Scholar] [CrossRef]
- Berger, F.; Gauvin, F.; Brouwers, H. The recycling potential of wood waste into wood-wool/cement composite. Construct Build. Mater. 2020, 260, 119786. [Google Scholar] [CrossRef]
- Hosseini, M.; Shahavi, M.H.; Yakhkeshi, A. AC & DC-currents for separation of nano-particles by external electric field. Asian J. Chem. 2012, 24, 181–184. [Google Scholar]
- de Klerk, S.; Ghaffariyan, M.R.; Miles, M. Leveraging the Entrepreneurial Method as a Tool for the Circular Economy: The Case of Wood Waste. Sustainability 2022, 14, 1559. [Google Scholar] [CrossRef]
- Che, X.; Wu, M.; Yu, G.; Liu, C.; Xu, H.; Li, B.; Li, C. Bio-inspired water resistant and fast multi-responsive Janus actuator assembled by cellulose nanopaper and graphene with lignin adhesion. Chem. Eng. J. 2021, 433, 133672. [Google Scholar] [CrossRef]
- Pizzi, A. Bioadhesives for wood and fibres. Rev. Adhes. Adhes. 2013, 1, 88–113. [Google Scholar] [CrossRef]
- Gaff, M.; Kačík, F.; Gašparík, M. Impact of thermal modification on the chemical changes and impact bending strength of European oak and Norway spruce wood. Compos. Struct. 2019, 216, 80–88. [Google Scholar] [CrossRef]
- Mohammadi-Rovshandeh, J.; Pouresmaeel-Selakjani, P.; Davachi, S.M.; Kaffashi, B.; Hassani, A.; Bahmeyi, A. Effect of lignin removal on mechanical, thermal, and morphological properties of polylactide/starch/rice husk blend used in food packaging. J. Appl. Polym. Sci. 2014, 131, 41095. [Google Scholar] [CrossRef]
- Hwang, S.W.; Shim, J.K.; Selke, S.; Soto-Valdez, H.; Rubino, M.; Auras, R. Effect of Maleic-Anhydride Grafting on the Physical and Mechanical Properties of Poly (L-lactic acid)/Starch Blends. Macromol. Mater. Eng. 2013, 298, 624–633. [Google Scholar] [CrossRef]
- ASTM D3418-21; Standard Test Method for Transition Temperatures and Enthalpies of Fusion and Crystallization of Polymers by Differential Scanning Calorimetry. ASTM International: West Conshohocken, PA, USA, 2021. [CrossRef]
- ASTM D638-14; Standard Test Method for Tensile Properties Of Plastics. ASTM International: West Conshohocken, PA, USA, 2014; p. 18. [CrossRef]
- ASTM D256-10; Standard Test Methods for Determining the Izod Pendulum Impact Resistance of Plastics. ASTM International: West Conshohocken, PA, USA, 2018. [CrossRef]
- ASTM D2240-15; Standard Test Method for Rubber Property—Durometer Hardness. ASTM International: West Conshohocken, PA, USA, 2021. [CrossRef]
- Abatari, M.N.; Emami, M.R.S.; Jahanshahi, M.; Shahavi, M.H. Superporous pellicular κ-Carrageenan–Nickel composite beads; morphological, physical and hydrodynamics evaluation for expanded bed adsorption application. Chem. Eng. Res. Des. 2017, 125, 291–305. [Google Scholar] [CrossRef]
- Rad, A.S.; Shahavi, M.H.; Esfahani, M.R.; Darvishinia, N.; Ahmadizadeh, S. Are nickel- and titanium- doped fullerenes suitable adsorbents for dopamine in an aqueous solution? Detailed DFT and AIM studies. J. Mol. Liq. 2021, 322, 114942. [Google Scholar] [CrossRef]
- Pérez Quiñones, J.; Brüggemann, O.; Kjems, J.; Shahavi, M.H.; Peniche Covas, C. Novel brassinosteroid-modified polyethylene glycol micelles for controlled release of agrochemicals. J. Agric. Food Chem. 2018, 66, 1612–1619. [Google Scholar] [CrossRef] [PubMed]
- Jahanshahi, M.; Shahavi, M.H. Chapter 17—Advanced Downstream Processing in Biotechnology. In Biochemical Engineering and Biotechnology, 2nd ed.; Najafpour, G.D., Ed.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 495–526. [Google Scholar]
- Sauerbier, P.; Köhler, R.; Renner, G.; Militz, H. Surface activation of polylactic acid-based wood-plastic composite by atmospheric pressure plasma treatment. Materials 2020, 13, 4673. [Google Scholar] [CrossRef]
- Lashkenari, A.S.; Najafi, M.; Peyravi, M.; Jahanshahi, M.; Mosavian, M.T.H.; Amiri, A.; Shahavi, M.H. Direct filtration procedure to attain antibacterial TFC membrane: A facile developing route of membrane surface properties and fouling resistance. Chem. Eng. Res. Des. 2019, 149, 158–168. [Google Scholar] [CrossRef]
- Shahavi, M.H.; Hosseini, M.; Jahanshahi, M.; Meyer, R.L.; Darzi, G.N. Clove oil nanoemulsion as an effective antibacterial agent: Taguchi optimization method. Desalination Water. Treat. 2016, 57, 18379–18390. [Google Scholar] [CrossRef]
- Halder, P.; Kundu, S.; Patel, S.; Parthasarathy, R.; Pramanik, B.; Paz-Ferreiro, J.; Shah, K. TGA-FTIR study on the slow pyrolysis of lignin and cellulose-rich fractions derived from imidazolium-based ionic liquid pre-treatment of sugarcane straw. Energy Convers. Manag. 2019, 200, 112067. [Google Scholar] [CrossRef]
- Hejna, A.; Barczewski, M.; Andrzejewski, J.; Kosmela, P.; Piasecki, A.; Szostak, M.; Kuang, T. Rotational molding of linear low-density polyethylene composites filled with wheat bran. Polymers 2020, 12, 1004. [Google Scholar] [CrossRef]
- Rahaman, M.N.; Brown, R.F. Materials for Biomedical Engineering: Fundamentals and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2021. [Google Scholar]
- Davachi, S.M.; Bakhtiari, S.; Pouresmaeel-Selakjani, P.; Mohammadi-Rovshandeh, J.; Kaffashi, B.; Davoodi, S.; Yousefi, A. Investigating the effect of treated rice straw in PLLA/starch composite: Mechanical, thermal, rheological, and morphological study. Adv. Polym. Technol. 2018, 37, 5–16. [Google Scholar] [CrossRef]
- Börcsök, Z.; Pásztory, Z. The role of lignin in wood working processes using elevated temperatures: An abbreviated literature survey. Eur. J. Wood Wood Prod. 2021, 79, 511–526. [Google Scholar] [CrossRef]
- Park, C.-W.; Youe, W.-J.; Kim, S.-J.; Han, S.-Y.; Park, J.-S.; Lee, E.-A.; Kwon, G.-J.; Kim, Y.-S.; Kim, N.-H.; Lee, S.-H. Effect of lignin plasticization on physico-mechanical properties of lignin/poly (lactic acid) composites. Polymers 2019, 11, 2089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrández-Montero, A.; Lieblich, M.; Benavente, R.; González-Carrasco, J.L.; Ferrari, B. Study of the matrix-filler interface in PLA/Mg composites manufactured by Material Extrusion using a colloidal feedstock. Addit. Manuf. 2020, 33, 101142. [Google Scholar] [CrossRef]
- Khan, T.A.; Lee, J.-H.; Kim, H.-J. Chapter 9—Lignin-Based Adhesives and Coatings. In Lignocellulose for Future Bioeconomy, Ariffin, H., Sapuan, S.M., Hassan, M.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 153–206. [Google Scholar]
- Nofar, M.; Sacligil, D.; Carreau, P.J.; Kamal, M.R.; Heuzey, M.-C. Poly (lactic acid) blends: Processing, properties and applications. Int. J. Biol. Macromol. 2019, 125, 307–360. [Google Scholar] [CrossRef]
- Tang, J.; Li, L.; Wang, X.; Yang, J.; Liang, X.; Li, Y.; Ma, H.; Zhou, S.; Wang, J. Tailored crystallization behavior, thermal stability, and biodegradability of poly (ethylene adipate): Effects of a biocompatible diamide nucleating agent. Polym. Test. 2020, 81, 106116. [Google Scholar] [CrossRef]
- Spinelli, G.; Guarini, R.; Kotsilkova, R.; Ivanov, E.; Romano, V. Experimental, Theoretical and Simulation Studies on the Thermal Behavior of PLA-Based Nanocomposites Reinforced with Different Carbonaceous Fillers. Nanomaterials 2021, 11, 1511. [Google Scholar] [CrossRef]
- Tábi, T. The application of the synergistic effect between the crystal structure of poly (lactic acid)(PLA) and the presence of ethylene vinyl acetate copolymer (EVA) to produce highly ductile PLA/EVA blends. J. Therm. Anal. Calorim. 2019, 138, 1287–1297. [Google Scholar] [CrossRef] [Green Version]
- Vanaei, H.R.; Shirinbayan, M.; Costa, S.F.; Duarte, F.M.; Covas, J.A.; Deligant, M.; Khelladi, S.; Tcharkhtchi, A. Experimental study of PLA thermal behavior during fused filament fabrication. J. Appl. Polym. Sci. 2021, 138, 49747. [Google Scholar] [CrossRef]
- Kachur, K.; Suntres, Z. The antibacterial properties of phenolic isomers, carvacrol and thymol. Crit. Rev. Food Sci. Nutr. 2020, 60, 3042–3053. [Google Scholar] [CrossRef]
- Sonseca, A.; Madani, S.; Rodríguez, G.; Hevilla, V.; Echeverría, C.; Fernández-García, M.; Muñoz-Bonilla, A.; Charef, N.; López, D. Multifunctional PLA blends containing chitosan mediated silver nanoparticles: Thermal, mechanical, antibacterial, and degradation properties. Nanomaterials 2020, 10, 22. [Google Scholar] [CrossRef] [Green Version]
- Chong, W.J.; Shen, S.; Li, Y.; Trinchi, A.; Pejak, D.; Kyratzis, I.L.; Sola, A.; Wen, C. Additive manufacturing of antibacterial PLA-ZnO nanocomposites: Benefits, limitations and open challenges. J. Mater. Sci. Technol. 2022, 111, 120–151. [Google Scholar] [CrossRef]








| Sample | PLA (%) | WL (%) | Mixing Temp (°C) | Mixing Time (min) | Sheet Thickness (mm) |
|---|---|---|---|---|---|
| PLA | 100 | 0 | 180 | 15 | 3.4 |
| PLA-WL-3 | 97 | 3 | 180 | 15 | 3.6 |
| PLA-WL-5 | 95 | 5 | 180 | 15 | 3.7 |
| PLA-WL-7 | 93 | 7 | 180 | 15 | 3.5 |
| PLA-WL-9 | 91 | 9 | 180 | 15 | 3.9 |
| Sample | Tensile Strength (MPa) | Elongation at Break (%) | Elastic Modulus (MPa) | Charpy Impact Strength (KJ/m2) | Shore A Hardness |
|---|---|---|---|---|---|
| PLA | 33.95 | 4.00 | 1837 | 38.62 | 89.21 |
| PLA-WL-3 | 58.65 | 8.80 | 1553 | 46.21 | 91.32 |
| PLA-WL-5 | 121.75 | 16.73 | 1817 | 62.47 | 91.85 |
| PLA-WL-7 | 169.75 | 18.93 | 1942 | 74.56 | 92.30 |
| PLA-WL-9 | 66.95 | 11.71 | 1427 | 61.84 | 86.21 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shahavi, M.H.; Selakjani, P.P.; Abatari, M.N.; Antov, P.; Savov, V. Novel Biodegradable Poly (Lactic Acid)/Wood Leachate Composites: Investigation of Antibacterial, Mechanical, Morphological, and Thermal Properties. Polymers 2022, 14, 1227. https://doi.org/10.3390/polym14061227
Shahavi MH, Selakjani PP, Abatari MN, Antov P, Savov V. Novel Biodegradable Poly (Lactic Acid)/Wood Leachate Composites: Investigation of Antibacterial, Mechanical, Morphological, and Thermal Properties. Polymers. 2022; 14(6):1227. https://doi.org/10.3390/polym14061227
Chicago/Turabian StyleShahavi, Mohammad Hassan, Peyman Pouresmaeel Selakjani, Mohadese Niksefat Abatari, Petar Antov, and Viktor Savov. 2022. "Novel Biodegradable Poly (Lactic Acid)/Wood Leachate Composites: Investigation of Antibacterial, Mechanical, Morphological, and Thermal Properties" Polymers 14, no. 6: 1227. https://doi.org/10.3390/polym14061227