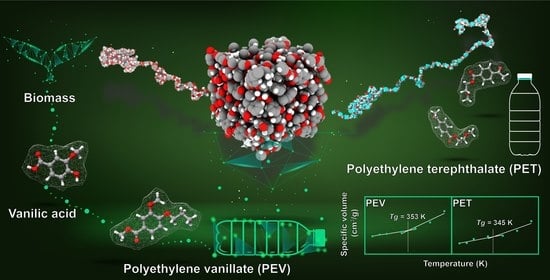
All-Atom Molecular Dynamics Simulations on a Single Chain of PET and PEV Polymers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Molecular Structures and Their Force Fields
2.2. MD Simulations
2.3. Structural Characterization
2.4. Prediction of Glass Transition Temperature
3. Results and Discussion
3.1. Folding of a Single-Chain Polymer
3.2. Structural Properties of Folded Polymer
3.2.1. The Total Radial Distribution Functions
3.2.2. The Site–Site Radial Distribution Functions
3.3. Dihedral Angle Distributions
3.4. Glass Transition Temperatures
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cole, M.; Lindeque, P.; Halsband, C.; Galloway, T.S. Microplastics as contaminants in the marine environment: A review. Mar. Pollut. Bull. 2011, 62, 2588–2597. [Google Scholar] [CrossRef]
- Schwarz, A.E.; Ligthart, T.N.; Boukris, E.; van Harmelen, T. Sources, transport, and accumulation of different types of plastic litter in aquatic environments: A review study. Mar. Pollut. Bull. 2019, 143, 92–100. [Google Scholar] [CrossRef]
- Sheavly, S.B.; Register, K.M. Marine Debris & Plastics: Environmental Concerns, Sources, Impacts and Solutions. J. Polym. Environ. 2007, 15, 301–305. [Google Scholar]
- Courtene-Jones, W.; Quinn, B.; Murphy, F.; Gary, S.F.; Narayanaswamy, B.E. Optimisation of enzymatic digestion and validation of specimen preservation methods for the analysis of ingested microplastics. Anal. Methods 2017, 9, 1437–1445. [Google Scholar] [CrossRef] [Green Version]
- Smith, M.; Love, D.C.; Rochman, C.M.; Neff, R.A. Microplastics in Seafood and the Implications for Human Health. Curr. Environ. Health Rep. 2018, 5, 375–386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halonen, N.; Pálvölgyi, P.S.; Bassani, A.; Fiorentini, C.; Nair, R.; Spigno, G.; Kordas, K. Bio-Based Smart Materials for Food Packaging and Sensors—A Review. Front. Mater. 2020, 7. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.J.; Rahman, A.T.M.M. Chapter 8—Intelligent Packaging for Food Products. In Innovations in Food Packaging (Second Edition); Han, J.H., Ed.; Academic Press: San Diego, CA, USA, 2014; pp. 171–209. [Google Scholar]
- Pang, C.; Zhang, J.; Wu, G.; Wang, Y.; Gao, H.; Ma, J. Renewable polyesters derived from 10-undecenoic acid and vanillic acid with versatile properties. Polym. Chem. 2014, 5. [Google Scholar] [CrossRef]
- Isikgor, F.H.; Becer, C.R. Lignocellulosic biomass: A sustainable platform for the production of bio-based chemicals and polymers. Polym. Chem. 2015, 6, 4497–4559. [Google Scholar] [CrossRef] [Green Version]
- Gioia, C.; Banella, M.B.; Marchese, P.; Vannini, M.; Colonna, M.; Celli, A. Advances in the synthesis of bio-based aromatic polyesters: Novel copolymers derived from vanillic acid and ε-caprolactone. Polym. Chem. 2016, 7, 5396–5406. [Google Scholar] [CrossRef]
- Zamboulis, A.; Papadopoulos, L.; Terzopoulou, Z.; Bikiaris, D.N.; Patsiaoura, D.; Chrissafis, K.; Gazzano, M.; Lotti, N.; Papageorgiou, G.Z. Synthesis, Thermal Properties and Decomposition Mechanism of Poly(Ethylene Vanillate) Polyester. Polymers 2019, 11, 1672. [Google Scholar] [CrossRef] [Green Version]
- Kayser, L.V.; Hartigan, E.M.; Arndtsen, B.A. Multicomponent Coupling Approach to Cross-Conjugated Polymers from Vanillin-Based Monomers. ACS Sustain. Chem. Eng. 2016, 4, 6263–6267. [Google Scholar] [CrossRef]
- Wilsens, C.H.R.M.; Verhoeven, J.M.G.A.; Noordover, B.A.J.; Hansen, M.R.; Auhl, D.; Rastogi, S. Thermotropic Polyesters from 2,5-Furandicarboxylic Acid and Vanillic Acid: Synthesis, Thermal Properties, Melt Behavior, and Mechanical Performance. Macromolecules 2014, 47, 3306–3316. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, H.; Deng, J.; Wu, Y. High Glass-Transition Temperature Acrylate Polymers Derived from Biomasses, Syringaldehyde, and Vanillin. Macromol. Chem. Phys. 2016, 217, 2402–2408. [Google Scholar] [CrossRef]
- Han, J.; Gee, R.H.; Boyd, R.H. Glass Transition Temperatures of Polymers from Molecular Dynamics Simulations. Macromolecules 1994, 27, 7781–7784. [Google Scholar] [CrossRef]
- Yang, Q.; Chen, X.; He, Z.; Lan, F.; Liu, H. The glass transition temperature measurements of polyethylene: Determined by using molecular dynamic method. RSC Adv. 2016, 6, 12053–12060. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian09, Revision B.01; Gaussian, Inc.: Wallingford, CT, USA, 2010. [Google Scholar]
- Sigfridsson, E.; Ryde, U. Comparison of methods for deriving atomic charges from the electrostatic potential and moments. J. Comput. Chem. 1998, 19, 377–395. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Maxwell, D.S.; Tirado-Rives, J. Development and Testing of the OPLS All-Atom Force Field on Conformational Energetics and Properties of Organic Liquids. J. Am. Chem. Soc. 1996, 118, 11225–11236. [Google Scholar] [CrossRef]
- Bernardes, C.E.S.; Joseph, A. Evaluation of the OPLS-AA Force Field for the Study of Structural and Energetic Aspects of Molecular Organic Crystals. J. Phys. Chem. A 2015, 119, 3023–3034. [Google Scholar] [CrossRef]
- Yong, C.W. Descriptions and Implementations of DL_F Notation: A Natural Chemical Expression System of Atom Types for Molecular Simulations. J. Chem. Inf. Modeling 2016, 56, 1405–1409. [Google Scholar] [CrossRef] [PubMed]
- Smith, W.; Yong, C.W.; Rodger, P.M. DL_POLY: Application to molecular simulation. Mol. Simul. 2002, 28, 385–471. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Li, M.; Liu, X.Y.; Qin, J.Q.; Gu, Y. Molecular dynamics simulation on glass transition temperature of isomeric polyimide. Express Polym. Lett. 2009, 3, 665–675. [Google Scholar] [CrossRef]
- Bejagam, K.K.; Iverson, C.N.; Marrone, B.L.; Pilania, G. Molecular dynamics simulations for glass transition temperature predictions of polyhydroxyalkanoate biopolymers. Phys. Chem. Chem. Phys. 2020, 22, 17880–17889. [Google Scholar] [CrossRef]
- Iñiguez, M.E.; Conesa, J.A.; Fullana, A. Microplastics in Spanish Table Salt. Sci. Rep. 2017, 7, 8620. [Google Scholar] [CrossRef] [PubMed]
- Karayiannis, N.C.; Mavrantzas, V.G.; Theodorou, D.N. Detailed Atomistic Simulation of the Segmental Dynamics and Barrier Properties of Amorphous Poly(ethylene terephthalate) and Poly(ethylene isophthalate). Macromolecules 2004, 37, 2978–2995. [Google Scholar] [CrossRef]
- Hedenqvist, M.S.; Bharadwaj, R.; Boyd, R.H. Molecular Dynamics Simulation of Amorphous Poly(ethylene terephthalate). Macromolecules 1998, 31, 1556–1564. [Google Scholar] [CrossRef]
- Giacobazzi, G.; Gioia, C.; Vannini, M.; Marchese, P.; Guillard, V.; Angellier-Coussy, H.; Celli, A. Water Vapor Sorption and Diffusivity in Bio-Based Poly(ethylene vanillate)—PEV. Polymers 2021, 13, 524. [Google Scholar] [CrossRef]
- Lepoittevin, B.; Roger, P. Poly(ethylene terephthalate). In Handbook of Engineering and Speciality Thermoplastics; Scrivener Publishing LLC: Beverly, MA, USA, 2011; pp. 97–126. [Google Scholar]








| Polymers | Glass Transition Temperature Tg (K) | |
|---|---|---|
| Simulations | Experiments | |
| PET | 345, 342 [11] | 350 [28], 353 [11,27] |
| PEV | 353 | 347 [10], 348 [29], 356 [11] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sangkhawasi, M.; Remsungnen, T.; Vangnai, A.S.; Poo-arporn, R.P.; Rungrotmongkol, T. All-Atom Molecular Dynamics Simulations on a Single Chain of PET and PEV Polymers. Polymers 2022, 14, 1161. https://doi.org/10.3390/polym14061161
Sangkhawasi M, Remsungnen T, Vangnai AS, Poo-arporn RP, Rungrotmongkol T. All-Atom Molecular Dynamics Simulations on a Single Chain of PET and PEV Polymers. Polymers. 2022; 14(6):1161. https://doi.org/10.3390/polym14061161
Chicago/Turabian StyleSangkhawasi, Mattanun, Tawun Remsungnen, Alisa S. Vangnai, Rungtiva P. Poo-arporn, and Thanyada Rungrotmongkol. 2022. "All-Atom Molecular Dynamics Simulations on a Single Chain of PET and PEV Polymers" Polymers 14, no. 6: 1161. https://doi.org/10.3390/polym14061161