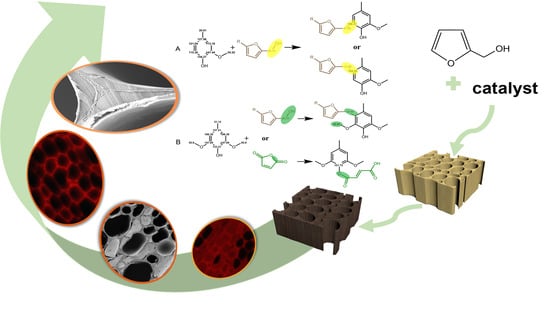
The Distribution and Polymerization Mechanism of Polyfurfuryl Alcohol (PFA) with Lignin in Furfurylated Wood
Abstract
:1. Introduction
2. Experimental Sections
2.1. Materials
2.2. Preparation of Furfurylated Wood
2.3. Preparation of Furfurylated Lignin
2.4. Characterization
3. Results and Discussion
3.1. Distribution of PFA Resin in Wood
3.2. The Polymerization Mechanism of PFA and CW
3.3. The Polymerization Mechanism of PFA and MWL
3.4. The Polymerization Mechanism of PFA and Lignin Model Molecules
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chheda, J.N.; Huber, G.W.; Dumesic, J.A. Liquid-phase catalytic processing of biomass-derived oxygenated hydrocarbons to fuels and chemicals. Angew. Chem. Int. Ed. 2007, 46, 7164–7183. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Altgen, M.; Mäkelä, M.; Rautkari, L.; Hughes, M.; Li, J.; Zhang, S. Improvement of interfacial interaction in impregnated wood via grafting methyl methacrylate onto wood cell walls. Holzforschung 2020, 74, 967–977. [Google Scholar] [CrossRef]
- Hill, C.; Hughes, M.; Gudsell, D. Environmental Impact of Wood Modification. Coatings 2021, 11, 366. [Google Scholar] [CrossRef]
- Furuno, T.; Imamura, Y.; Kajita, H. The modification of wood by treatment with low molecular weight phenol-formaldehyde resin: A properties enhancement with neutralized phenolic-resin and resin penetration into wood cell walls. Wood Sci. Technol. 2004, 37, 349–361. [Google Scholar] [CrossRef]
- Jiang, Y.F.; Wu, G.F.; Chen, H.Y.; Song, S.P.; Pu, J.W. Preparation of nano-SiO2 modified urea-formaldehyde performed polymer to enhance wood properties. Rev. Adv. Mater. Sci. 2013, 33, 46–50. [Google Scholar]
- Deka, M.; Gindl, W.; Wimmer, R.; Christian, H. Chemical modification of Norway spruce (Picea abies (L) Karst) wood with melamine formaldehyde resin. Indian J. Chem. Technol. 2007, 14, 134–138. [Google Scholar]
- Li, W.J.; Ren, D.; Zhang, X.X.; Wang, H.K.; Yu, Y. The Furfurylation of Wood: A Nanomechanical Study of Modified Wood Cells. Bioresources 2016, 11, 3614–3625. [Google Scholar] [CrossRef]
- Lande, S.; Eikenes, M.; Westin, M. Chemistry and ecotoxicology of furfurylated wood. Scand. J. For. Res. 2004, 19, 14–21. [Google Scholar] [CrossRef]
- Keplinger, T.; Cabane, E.; Chanana, M.; Hass, P.; Merk, V.; Gierlinger, N.; Burgert, I. A versatile strategy for grafting polymers to wood cell walls. Acta. Biomater. 2015, 11, 256–263. [Google Scholar] [CrossRef]
- Yang, T.; Ma, E.; Cao, J. Synergistic effects of partial hemicellulose removal and furfurylation on improving the dimensional stability of poplar wood tested under dynamic condition. Ind. Crops Prod. 2019, 139, 111550. [Google Scholar] [CrossRef]
- Thygesen, L.G.; Barsberg, S.; Venås, T.M. The fluorescence characteristics of furfurylated wood studied by fluorescence spectroscopy and confocal laser scanning microscopy. Wood Sci. Technol. 2009, 44, 51–65. [Google Scholar] [CrossRef]
- Yang, T.; Cao, J.; Ma, E. How does delignification influence the furfurylation of wood? Ind. Crops Prod. 2019, 135, 91–98. [Google Scholar] [CrossRef]
- Ehmcke, G.; Pilgård, A.; Koch, G.; Richter, K. Topochemical analyses of furfuryl alcohol-modified radiata pine (Pinus radiata) by UMSP, light microscopy and SEM. Holzforschung 2017, 71, 821–831. [Google Scholar] [CrossRef] [Green Version]
- Nordstierna, L.; Lande, S.; Westin, M.; Karlsson, O.; Furo, I. Towards novel wood-based materials: Chemical bonds between lignin-like model molecules and poly(furfuryl alcohol) studied by NMR. Holzforschung 2008, 62, 709–713. [Google Scholar] [CrossRef]
- Fu, Q.L.; Ansari, F.; Zhou, Q.; Berglund, L.A. Wood Nanotechnology for Strong, Mesoporous, and Hydrophobic Biocomposites for Selective Separation of Oil/Water Mixtures. ACS Nano 2018, 12, 2222–2230. [Google Scholar] [CrossRef]
- BjÖRkman, A. Isolation of Lignin from Finely Divided Wood with Neutral Solvents. Nature 1954, 174, 1057–1058. [Google Scholar] [CrossRef]
- Tarmian, A.; Zahedi Tajrishi, I.; Oladi, R.; Efhamisisi, D. Treatability of wood for pressure treatment processes: A literature review. Eur. J. Wood Wood Prod. 2020, 78, 635–660. [Google Scholar] [CrossRef]
- Jin, K.; Liu, X.; Wang, K.; Jiang, Z.; Tian, G.; Yang, S.; Shang, L.; Ma, J. Imaging the dynamic deposition of cell wall polymer in xylem and phloem in Populus x euramericana. Planta 2018, 248, 849–858. [Google Scholar] [CrossRef]
- Saariaho, A.M.; Jaaskelainen, A.S.; Nuopponen, M.; Vuorinen, T. Ultra violet resonance Raman spectroscopy in lignin analysis: Determination of characteristic vibrations of p-hydroxyphenyl, guaiacyl, and syringyl lignin structures. Appl. Spectrosc. 2003, 57, 58–66. [Google Scholar] [CrossRef]
- Agarwal, U.P. Raman imaging to investigate ultrastructure and composition of plant cell walls: Distribution of lignin and cellulose in black spruce wood (Picea mariana). Planta 2006, 224, 1141–1153. [Google Scholar] [CrossRef]
- Larsen, K.L.; Barsberg, S. Theoretical and Raman spectroscopic studies of phenolic lignin model monomers. J. Phys. Chem. B 2010, 114, 8009–8021. [Google Scholar] [CrossRef] [PubMed]
- Dolk, M.; Yan, J.F.; Mccarthy, J.L. Lignin-25—Kinetics of Delignification of Western Hemlock in Flow-through Reactors under Alkaline Conditions. Holzforschung 1989, 43, 91–98. [Google Scholar] [CrossRef]
- Brogdon, B.N.; Mancosky, D.G.; Lucia, L.A. New Insights into Lignin Modification During Chlorine Dioxide Bleaching Sequences (II): Modifications in Extraction (E) and Chlorine Dioxide Bleaching (D1). J. Wood Chem. Technol. 2005, 24, 221–237. [Google Scholar] [CrossRef]
- Meng, Y.; Majoinen, J.; Zhao, B.; Rojas, O.J. Form-stable phase change materials from mesoporous balsa after selective removal of lignin. Compos. Part B 2020, 199, 108296. [Google Scholar] [CrossRef]
- Korotkova, E.; Pranovich, A.; Wärnå, J.; Salmi, T.; Murzin, D.Y.; Willför, S. Lignin isolation from spruce wood with low concentration aqueous alkali at high temperature and pressure: Influence of hot-water pre-extraction. Green Chem. 2015, 17, 5058–5068. [Google Scholar] [CrossRef]
- Lems, E.-M.; Winklehner, S.; Hansmann, C.; Gindl-Altmutter, W.; Veigel, S. Reinforcing effect of poly(furfuryl alcohol) in cellulose-based porous materials. Cellulose 2019, 26, 4431–4444. [Google Scholar] [CrossRef] [Green Version]
- Jahan, M.S.; Mun, S.P. Characteristics of milled wood lignins isolated from different ages of nalita wood (Trema orientalis). Cell Chem. Technol. 2006, 40, 457–467. [Google Scholar]
- Alves, A.; Santos, S.; Simoes, R.; Rodrigues, J. Characterization of residual lignin in cellulose isolated by the diglyme method from three Pinus species by IR spectroscopy and analytical pyrolysis. Holzforschung 2018, 72, 91–96. [Google Scholar] [CrossRef]
- Faix, O. Classification of Lignins from Different Botanical Origins by FT-IR Spectroscopy. Holzforschung 1991, 45, 21–28. [Google Scholar] [CrossRef]
- Holtman, K.M.; Chang, H.M.; Kadla, J.F. Solution-state nuclear magnetic resonance study of the similarities between milled wood lignin and cellulolytic enzyme lignin. J. Agric. Food Chem. 2004, 52, 720–726. [Google Scholar] [CrossRef]
- Pang, B.; Yang, S.; Fang, W.; Yuan, T.-Q.; Argyropoulos, D.S.; Sun, R.-C. Structure-property relationships for technical lignins for the production of lignin-phenol-formaldehyde resins. Ind. Crops Prod. 2017, 108, 316–326. [Google Scholar] [CrossRef]
- Jacobs, A. Understanding Organic Reaction Mechanisms; Cambridge University Press: Cambridge, UK, 1997. [Google Scholar] [CrossRef]
- Barsberg, S.T.; Thygesen, L.G. A Combined Theoretical and FT-IR Spectroscopy Study of a Hybrid poly(furfuryl alcohol)—Lignin Material: Basic Chemistry of a Sustainable Wood Protection Method. ChemistrySelect 2017, 2, 10818–10827. [Google Scholar] [CrossRef]
- Guigo, N.; Mija, A.; Vincent, L.; Sbirrazzuoli, N. Eco-friendly composite resins based on renewable biomass resources: Polyfurfuryl alcohol/lignin thermosets. Eur. Polym. J. 2010, 46, 1016–1023. [Google Scholar] [CrossRef]






| Samples | Infiltration Area (μm2) | Ratio of Permeable Area (%) |
|---|---|---|
| CW | 4.10 | 6.07 |
| dyed CW | 2.96 | 4.39 |
| 10% FA CFW | 69,484.04 | 22.19 |
| 20% FA CFW | 84,619.40 | 27.02 |
| 30% FA CFW | 93,675.93 | 29.91 |
| 40% FA CFW | 106,763.16 | 34.09 |
| 50% FA CFW | 110,196.67 | 35.19 |
| 50% FA DFW | 210,576.16 | 67.24 |
| Peak | δ | Assignment | Peak | δ | Assignment |
|---|---|---|---|---|---|
| 1 | 170.91 | C=O inα | 8 | 114.19 | C3/C5 in G’ |
| 2 | 152.99 | C3/C5 in S | 9 | 103.62 | C2/C6 in S |
| 3 | 147.30 | C3/C5 in S’ | 10 | 81.63 | Cβ in III, Cα in I |
| 4 | 135.36 | C1 in G | 11 | 73.92 | Cα in III |
| 5 | 134.11 | C1 in G’ | 12 | 55.38 | -OCH3 |
| 6 | 121.01 | C6 in G | 13 | 20.00 | -CH3 |
| 7 | 115.70 | C3/C5 in G | —— | —— | —— |
| Peak | δ | Assignment |
|---|---|---|
| 1 | 207.28 | C=O in Furan ring opening |
| 2 | 170.69 | C=O in p-Coumaric acid |
| 3 | 151.62 | C1 in chain structure of PFA, C3/C5 in S |
| 4 | 142.67 | C4 in chain structure of PFA |
| 5 | 134.58 | C1 in G/G’ |
| 6 | 108.15 | C3 in chain structure of PFA |
| 7 | 55.41 | -OCH3 in MWL |
| 8 | 37.79 | -CH2 in reticular conformation of PFA |
| 9 | 27.38 | -CH2 in chain structure of PFA |
| 10 | 20.46 | -CH3 in MWL |
| 11 | 13.18 | -CH3 in chain structure of PFA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Hu, D.; Zheng, Q.; Meng, Q.; Li, N. The Distribution and Polymerization Mechanism of Polyfurfuryl Alcohol (PFA) with Lignin in Furfurylated Wood. Polymers 2022, 14, 1071. https://doi.org/10.3390/polym14061071
Xu J, Hu D, Zheng Q, Meng Q, Li N. The Distribution and Polymerization Mechanism of Polyfurfuryl Alcohol (PFA) with Lignin in Furfurylated Wood. Polymers. 2022; 14(6):1071. https://doi.org/10.3390/polym14061071
Chicago/Turabian StyleXu, Jindi, Dongying Hu, Qi Zheng, Qiulu Meng, and Ning Li. 2022. "The Distribution and Polymerization Mechanism of Polyfurfuryl Alcohol (PFA) with Lignin in Furfurylated Wood" Polymers 14, no. 6: 1071. https://doi.org/10.3390/polym14061071