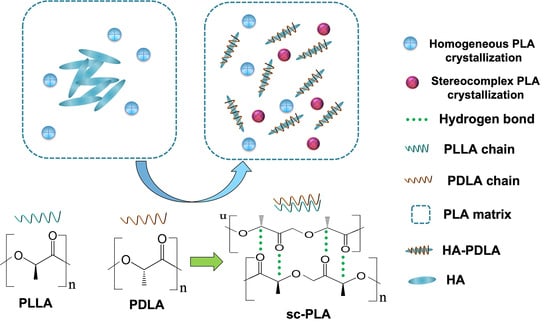
Stereocomplexation Reinforced High Strength Poly(L-lactide)/Nanohydroxyapatite Composites for Potential Bone Repair Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Surface Modification of n-HA
2.3. Solution Casting of mHA1–3/PLA Nanocomposite Films
2.4. Characterizations
2.4.1. Scanning Electron Microscopy (SEM)
2.4.2. Fourier Transform Infrared Spectroscopy (FT-IR)
2.4.3. X-ray Diffraction (XRD)
2.4.4. Thermogravimetric Analysis (TGA)
2.4.5. Dynamic Light Scattering (DLS)
2.4.6. Polarized Optical Microscopy (POM)
2.4.7. Tensile Testing
2.4.8. Differential Scanning Calorimetry (DSC)
2.4.9. Cell Seeding
2.4.10. Hemolytic Property of HA
2.4.11. Cytotoxicity of the HA/mHA
2.4.12. Cell Proliferation on mHA/PLA Composites
2.4.13. Alizarin Red S (ARS) Staining and Quantification
2.4.14. ALP Activity Measurement
2.4.15. Osteocalcin (OCN) Immunofluorescence Staining
2.4.16. Confocal Laser Scanning Microscopy (CLSM)
2.4.17. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Appendix A
- Silanization of reaction vessel
- 2.
- Synthesis of schistose n-HA

References
- Régibeau, N.; Tilkin, G.; Grandfils, C.; Heinrichs, B. Preparation of Poly-D, L-lactide Based Nanocomposites with Polymer-grafted Silica by Melt Blending: Study of Molecular, Morphological, and Mechanical Propertie. Polym. Compos. 2021, 42, 955–972. [Google Scholar] [CrossRef]
- Fortunati, E.; Luzi, F.; Puglia, D.; Petrucci, R.; Kenny, J.M.; Torre, L. Processing of PLA Nanocomposites with Cellulose Nanocrystals Extracted from Posidonia Oceanica Waste: Innovative Reuse of Coastal Plant. Ind. Crop. Prod. 2015, 67, 439–447. [Google Scholar] [CrossRef]
- Agrawal, M.; Ray, B. Biodegradable Polymeric Scaffolds for Musculoskeletal Tissue Engineering. J. Biomed. Mater. Res. 2001, 55, 141–150. [Google Scholar] [CrossRef]
- Hrubovcakova, M.; Kupkova, M.; Dzupon, M.; Giretova, M.; Medvecky, L.; Dzunda, M. Biodegradable Polylactic Acid and Polylactic Acid/Hydroxyapatite Coated Iron Foams for Bone Replacement Material. Int. J. Electrochem. Sci. 2017, 12, 11122–11136. [Google Scholar] [CrossRef]
- Shady, F.; Daniel, G.A.; Robert, L. Physical and mechanical properties of PLA, and their functions in widespread applications -A comprehensive review. Adv. Drug Deliv. Rev. 2016, 107, 367–392. [Google Scholar]
- Oh, S.H.; Lee, J.H. Hydrophilization of Synthetic Biodegradable Polymer Scaffolds for Improved Cell/Tissue Compatibility. Biomed. Mater. 2013, 8, 014101. [Google Scholar] [CrossRef] [PubMed]
- Rasal, M.; Janorkar, A.V.; Hirt, D.E. Poly(Lactic Acid) Modification. Prog. Polym. Sci. 2010, 35, 338–356. [Google Scholar] [CrossRef]
- Bohner, M.; Miron, J. A Proposed Mechanism for Material-Induced Heterotopic Ossification. Mater. Today 2019, 22, 132–141. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, B.; Li, M.; Li, J.; Zhang, H.Y.; Wang, L.; Wang, K.; Zhou, L.L.; Fan, Y.; Zhang, X. 3D Printing of PLA/n-HA Composite Scaffolds with Customized Mechanical Properties and Biological Functions for Bone Tissue Engineering. Compos. Part B Eng. 2021, 224, 109192. [Google Scholar] [CrossRef]
- Cao, H.; Kuboyama, N. A Biodegradable Porous Composite Scaffold of PGA/Beta-TCP for Bone Tissue Engineering. Bone 2010, 46, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Dorozhkin, S.V. Calcium Orthophosphate Coatings on Magnesium and Its Biodegradable Alloy. Acta Biomater. 2014, 10, 2919–2934. [Google Scholar] [CrossRef]
- Fu, S.; Ni, P.; Wang, B.; Chu, B.; Zheng, L.; Luo, F.; Luo, J.; Qian, Z. Injectable and Thermo-Sensitive PEG-PCL-PEG Copolymer/Collagen/n-HA Hydrogel Composite for Guided Bone Regeneration. Biomaterials 2012, 33, 4801–4809. [Google Scholar] [CrossRef] [PubMed]
- Ali, W.; Mehboob, A.; Han, M.-G.; Chang, S.-H. Novel Biodegradable Hybrid Composite of Polylactic Acid (PLA) Matrix Reinforced by Bioactive Glass (BG) Fibres and Magnesium (Mg) Wires for Orthopaedic Application. Compos. Struct. 2020, 245, 112322. [Google Scholar] [CrossRef]
- Barradas, A.M.; Fernandes, H.A.M.; Groen, N.; Chai, Y.; Schrooten, J.; van de Peppel, J.; van Leeuwen, J.P.T.M.; van Blitterswijk, A.; de Boer, J. A Calcium-Induced Signaling Cascade Leading to Osteogenic Differentiation of Human Bone Marrow-Derived Mesenchymal Stromal Cell. Biomaterials 2012, 33, 3205–3215. [Google Scholar] [CrossRef] [PubMed]
- Shih, Y.-V.; Hwang, Y.; Phadke, A.; Kang, H.; Hwang, N.S.; Caro, E.J.; Nguyen, S.; Siu, M.; Theodorakis, E.A.; Gianneschi, N.; et al. Calcium Phosphate-Bearing Matrices Induce Osteogenic Differentiation of Stem Cells through Adenosine Signaling. Proc. Natl. Acad. Sci. 2014, 111, 990–995. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.-K.; Yu, X.; Cohen, D.M.; Wozniak, M.A.; Yang, M.T.; Gao, L.; Eyckmans, J.; Chen, S. Bone Morphogenetic Protein-2-Induced Signaling and Osteogenesis Is Regulated by Cell Shape, RhoA/ROCK, and Cytoskeletal Tension. Stem Cells Dev. 2012, 21, 1176–1186. [Google Scholar] [CrossRef] [Green Version]
- Lian, J.B.; Stein, G.S.; Javed, A.; van Wijnen, A.J.; Stein, J.L.; Montecino, M.; Hassan, M.Q.; Gaur, T.; Lengner, J.; Young, D.W. Networks and Hubs for the Transcriptional Control of Osteoblastogenesi. Rev. Endocr Metab Disord 2006, 7, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Okada, S.; Ito, H.; Nagai, A.; Komotori, J.; Imai, H. Adhesion of Osteoblast-like Cells on Nanostructured Hydroxyapatit. Acta Biomater. 2010, 6, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Ricotti, L.; Taccola, S.; Pensabene, V.; Mattoli, V.; Fujie, T.; Takeoka, S.; Menciassi, A.; Dario, P. Adhesion and Proliferation of Skeletal Muscle Cells on Single Layer Poly(Lactic Acid) Ultra-Thin Film. Biomed. Microdevices 2010, 12, 809–819. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Zhou, C.; Hong, Y.; Zhang, X. A Review of Protein Adsorption on Bioceramic. Interface Focu 2012, 2, 259–277. [Google Scholar] [CrossRef]
- Xu, X.; Chen, X.; Liu, A.; Hong, Z.; Jing, X. Electrospun Poly(L-Lactide)-Grafted Hydroxyapatite/Poly(L-Lactide) Nanocomposite Fiber. Eur. Polym. J. 2007, 43, 3187–3196. [Google Scholar] [CrossRef]
- Toledano, M.; Osorio, R.; Vallecillo-Rivas, M.; Osorio, E.; Lynch, D.; Aguilera, F.S.; Toledano, R.; Sauro, S. Zn-Doping of Silicate and Hydroxyapatite-Based Cements: Dentin Mechanobiology and Bioactivity. J. Mech. Behav. Biomed. Mater. 2021, 114, 104232. [Google Scholar] [CrossRef] [PubMed]
- Fernando, D.; Attik, N.; Pradelle-Plasse, N.; Jackson, P.; Grosgogeat, B.; Colon, P. Bioactive Glass for Dentin Remineralization: A Systematic Review. Mater. Sci. Eng. C 2017, 76, 1369–1377. [Google Scholar] [CrossRef] [PubMed]
- Bartos, A.; Nagy, K.; Anggono, J.; Antoni Purwaningsih, H.; Móczó, J.; Pukánszky, B. Biobased PLA/Sugarcane Bagasse Fiber Composites: Effect of Fiber Characteristics and Interfacial Adhesion on Propertie. Compos. Part A Appl. Sci. Manuf. 2021, 143, 106273. [Google Scholar] [CrossRef]
- Chuan, D.; Fan, R.; Wang, Y.; Ren, Y.; Wang, C.; Du, Y.; Zhou, L.; Yu, J.; Gu, Y.; Chen, H.; et al. Stereocomplex Poly(Lactic Acid)-Based Composite Nanofiber Membranes with Highly Dispersed Hydroxyapatite for Potential Bone Tissue Engineering. Compos. Sci. Technol. 2020, 192, 108107. [Google Scholar] [CrossRef]
- Rocha, C.R.; Chávez-Flores, D.; Zuverza-Mena, N.; Duarte, A.; Rocha-Gutiérrez, B.A.; Zaragoza-Contreras, E.A.; Flores-Gallardo, S. Surface Organo-modification of Hydroxyapatites to Improve PLA/HA Compatibility. J. Appl Polym Sci 2020, 137, 49293. [Google Scholar] [CrossRef]
- Fang, Z.; Feng, Q. Improved Mechanical Properties of Hydroxyapatite Whisker-Reinforced Poly(L-Lactic Acid) Scaffold by Surface Modification of Hydroxyapatit Mater. Sci. Eng. C 2014, 35, 190–194. [Google Scholar] [CrossRef]
- Wang, X.; Song, G.; Lou, T. Fabrication and Characterization of Nano-Composite Scaffold of PLLA/Silane Modified Hydroxyapatit. Med Eng. Phys. 2010, 32, 391–397. [Google Scholar] [CrossRef]
- Tsuji, H.; Ikada, Y. Stereocomplex Formation between Enantiomeric Poly(Lactic Acids). 9. Stereocomplexation from the Melt. Macromolecules 1993, 26, 6918–6926. [Google Scholar] [CrossRef]
- Nakajima, M.; Nakajima, H.; Fujiwara, T.; Kimura, Y.; Sasaki, S. Nano-Ordered Surface Morphologies by Stereocomplexation of the Enantiomeric Polylactide Chains: Specific Interactions of Surface-Immobilized Poly(D-Lactide) and Poly(Ethylene Glycol)-Poly(L-Lactide) Block Copolymer. Langmuir 2014, 30, 14030–14038. [Google Scholar] [CrossRef]
- Fan, X.; Wang, M.; Yuan, D.; He, C. Amphiphilic Conetworks and Gels Physically Cross-Linked via Stereocomplexation of Polylactid. Langmuir 2013, 29, 14307–14313. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, H.; Matsumura, N.; Arakawa, Y. Stereocomplex Crystallization and Homo-Crystallization of Star-Shaped Four-Armed Stereo Diblock Poly(Lactide)s during Precipitation and Non-Isothermal Crystallization. Polym J. 2016, 48, 1087–1093. [Google Scholar] [CrossRef]
- Tsuji, H.; Ikada, Y.; Hyon, S.-H.; Kimura, Y.; Kitao, T. Stereocomplex Formation between Enantiomeric Poly(Lactic Acid). VIII. Complex Fibers Spun from Mixed Solution of Poly(D-Lactic Acid) and Poly(L-Lactic Acid). J. Appl. Polym. Sci. 1994, 51, 337–344. [Google Scholar] [CrossRef]
- Tsuji, H.; Tezuka, Y. Stereocomplex Formation between Enantiomeric Poly(Lactic Acid) 12. Spherulite Growth of Low-Molecular-Weight Poly(Lactic Acid)s from the Melt. Biomacromolecules 2004, 5, 1181–1186. [Google Scholar] [CrossRef] [PubMed]
- Bai, D.; Liu, H.; Ju, Y.; Deng, S.; Bai, H.; Zhang, Q.; Fu, Q. Low-Temperature Sintering of Stereocomplex-Type Polylactide Nascent Powder: The Role of Poly(Methyl Methacrylate) in Tailoring the Interfacial Crystallization between Powder Particle. Polymer 2020, 210, 123031. [Google Scholar] [CrossRef]
- Fernandes Nassar, S.; Delpouve, N.; Sollogoub, C.; Guinault, A.; Stoclet, G.; Régnier, G.; Domenek, S. Impact of Nanoconfinement on Polylactide Crystallization and Gas Barrier Propertie. ACS Appl. Mate Interfaces 2020, 12, 9953–9965. [Google Scholar] [CrossRef]
- Jing, Y.; Quan, C.; Liu, B.; Jiang, Q.; Zhang, A. Mini Review on the Functional Biomaterials Based on Poly(Lactic Acid) Stereocomplex. Polym. Rev. 2016, 56, 262–286. [Google Scholar] [CrossRef]
- Kum, H.; Cho, Y.; Seo, S.H.; Joung, Y.K.; Ahn, D.J.; Han, D.K. A Poly(Lactide) Stereocomplex Structure with Modified Magnesium Oxide and Its Effects in Enhancing the Mechanical Properties and Suppressing Inflammation. Small 2014, 10, 3783–3794. [Google Scholar] [CrossRef]
- Jing, Z.; Shi, X.; Zhang, G.; Li, J. Rheology and Crystallization Behavior of PLLA/TiO2-g-PDLA Composites: Rheology and Crystallization Behavior of PLLA/TiO2-g-PDLA Composite. Polym. Adv. Technol. 2015, 26, 528–537. [Google Scholar] [CrossRef]
- Li, Z.; Yuan, D.; Jin, G.; Tan, B.H.; He, C. Facile Layer-by-Layer Self-Assembly toward Enantiomeric Poly(Lactide) Stereocomplex Coated Magnetite Nanocarrier for Highly Tunable Drug Deliverie. ACS Appl. Mate Interfaces 2016, 8, 1842–1853. [Google Scholar] [CrossRef]
- Li, Y.; Han, C.; Zhang, X.; Xu, K.; Bian, J.; Dong, L. Poly(L-Lactide)/Poly(D-Lactide)/Clay Nanocomposites: Enhanced Dispersion, Crystallization, Mechanical Properties, and Hydrolytic Degradation. Polym. Eng. Sci. 2014, 54, 914–924. [Google Scholar] [CrossRef]
- Huang, G.; Du, Z.; Yuan, Z.; Gu, L.; Cai, Q.; Yang, X. Poly(L-Lactide) Nanocomposites Containing Poly(D-Lactide) Grafted Nanohydroxyapatite with Improved Interfacial Adhesion via Stereocomplexation. J. Mech. Behav. Biomed. Mater. 2018, 78, 10–19. [Google Scholar] [CrossRef]
- Liu, C.; Lin, C.; Feng, X.; Wu, Z.; Lin, G.; Quan, C.; Chen, B.; Zhang, C. A Biomimicking Polymeric Cryogel Scaffold for Repair of Critical-Sized Cranial Defect in a Rat Model. Tissue Eng. Part A 2019, 25, 1591–1604. [Google Scholar] [CrossRef]
- Nogueira, D.; Scheeren, L.E.; Pilar Vinardell, M.; Mitjans, M.; Rosa Infante, M.; Rolim, M.B. Nanoparticles Incorporating PH-Responsive Surfactants as a Viable Approach to Improve the Intracellular Drug Delivery. Mater. Sci. Eng. C 2015, 57, 100–106. [Google Scholar] [CrossRef] [Green Version]
- Chu, L.; Jiang, G.; Hu, X.-L.; James, T.D.; He, X.-P.; Li, Y.; Tang, T. Biodegradable Macroporous Scaffold with Nano-Crystal Surface Microstructure for Highly Effective Osteogenesis and Vascularization. J. Mate Chem. B 2018, 6, 1658–1667. [Google Scholar] [CrossRef] [Green Version]
- Razavi, M.; Cheng, S.; Huang, D.; Zhang, S.; Wang, S.-Q. Crazing and Yielding in Glassy Polymers of High Molecular Weight. Polymer 2020, 197, 122445. [Google Scholar] [CrossRef]
- Deblieck, A.; van Beek, D.J.M.; Remerie, K.; Ward, I.M. Failure Mechanisms in Polyolefines: The Role of Crazing, Shear Yielding and the Entanglement Network. Polymer 2011, 52, 2979–2990. [Google Scholar] [CrossRef] [Green Version]
- Razavi, M.; Wang, S.-Q. Why Is Crystalline Poly(Lactic Acid) Brittle at Room Temperature? Macromolecules 2019, 52, 5429–5441. [Google Scholar] [CrossRef]
- Xu, J.-Z.; Zhang, Z.-J.; Xu, H.; Chen, J.-B.; Ran, R.; Li, Z.-M. Highly Enhanced Crystallization Kinetics of Poly(L-Lactic Acid) by Poly(Ethylene Glycol) Grafted Graphene Oxide Simultaneously as Heterogeneous Nucleation Agent and Chain Mobility Promote. Macromolecules 2015, 48, 4891–4900. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.N.; Huang, Z.G.; Weng, Y.X. Heat resistance, crystallization behavior, and mechanical properties of polylactide/nucleating agent composite. Mater. Des. 2015, 66, 7–15. [Google Scholar] [CrossRef]
- Baldenegro-Perez, L.A.; Navarro-Rodriguez, D.; Medellin-Rodriguez, F.J.; Hsiao, B.; Avila-Orta, A.; Sics, I. Molecular Weight and Crystallization Temperature Effects on Poly(ethylene terephthalate) (PET) Homopolymers, an Isothermal Crystallization Analysi. Polymers 2014, 6, 583–600. [Google Scholar] [CrossRef] [Green Version]













| Samples | Tg (°C) | Tcc (°C) | Tm (°C) | ΔHm (J/g) | ΔHcc (J/g) | Xc (%) |
|---|---|---|---|---|---|---|
| Neat PLLA mHA1/PLA | 58.3 63.0 | 122.8 108.7 | 178.4 179.6 | 37.6 44.5 | −21.1 −13.0 | 17.8 34.3 |
| mHA2/PLA | 61.8 | 105.4 | 179.3 | 45.3 | −12.6 | 35.6 |
| mHA3/PLA | 62.6 | 108.2 | 180.1 | 42.4 | −11.4 | 33.7 |
| Samples | 1.25 °C/min | 2.50 °C/min | 5.00 °C/min | 7.50 °C/min | 10.00 °C/min |
|---|---|---|---|---|---|
| PLLA | 19.16 | 11.87 | 7.31 | 4.08 | 2.39 |
| mHA1/PLA | 16.43 | 10.14 | 6.16 | 3.56 | 2.00 |
| mHA2/PLA | 15.62 | 9.27 | 5.98 | 3.48 | 1.87 |
| mHA3/PLA | 14.99 | 9.15 | 5.51 | 3.15 | 1.64 |
| Samples | Avrami | 1.25 °C/min | 2.50 °C/min | 5.00 °C/min | 7.50 °C/min | 10.00 °C/min |
|---|---|---|---|---|---|---|
| Neat PLLA | n1 | 3.61 | 4.44 | 5.43 | 6.65 | 6.93 |
| Zc1 | 4.29 | 4.25 | 4.05 | 3.25 | 1.8 | |
| n2 | 1.15 | 2.06 | 2.78 | 3.27 | 4.24 | |
| Zc2 | 1.19 | 1.12 | 0.98 | 0.84 | 0.57 | |
| mHA1/PLA | n1 | 3.41 | 3.96 | 4.66 | 6.35 | 8.17 |
| Zc1 | 4.54 | 4.42 | 4.2 | 3.94 | 2.62 | |
| n2 | 1.42 | 1.69 | 1.9 | 2.52 | 3.15 | |
| Zc2 | 1.62 | 1.6 | 1.31 | 1.29 | 0.85 | |
| mHA2/PLA | n1 | 3.2 | 3.93 | 4.77 | 6.29 | 8.49 |
| Zc1 | 4.42 | 4.5 | 4.16 | 3.85 | 2.6 | |
| n2 | 1.13 | 1.29 | 2.08 | 2.52 | 2.85 | |
| Zc2 | 1.23 | 0.95 | 1.54 | 1.29 | 0.68 | |
| mHA3/PLA | n1 | 3.59 | 4.43 | 4.14 | 4.98 | 5.38 |
| Zc1 | 4.62 | 4.55 | 3.38 | 2.76 | 1.31 | |
| n2 | 1.11 | 1.7 | 1.93 | 2.14 | 2.5 | |
| Zc2 | 1.21 | 1.6 | 1.38 | 0.98 | 0.53 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, N.; Zhao, M.; Li, S.; Hao, J.; Wu, Z.; Zhang, C. Stereocomplexation Reinforced High Strength Poly(L-lactide)/Nanohydroxyapatite Composites for Potential Bone Repair Applications. Polymers 2022, 14, 645. https://doi.org/10.3390/polym14030645
Guo N, Zhao M, Li S, Hao J, Wu Z, Zhang C. Stereocomplexation Reinforced High Strength Poly(L-lactide)/Nanohydroxyapatite Composites for Potential Bone Repair Applications. Polymers. 2022; 14(3):645. https://doi.org/10.3390/polym14030645
Chicago/Turabian StyleGuo, Naishun, Mengen Zhao, Sijing Li, Jiahui Hao, Zhaoying Wu, and Chao Zhang. 2022. "Stereocomplexation Reinforced High Strength Poly(L-lactide)/Nanohydroxyapatite Composites for Potential Bone Repair Applications" Polymers 14, no. 3: 645. https://doi.org/10.3390/polym14030645