Polyurethane Wood Adhesives Prepared from Modified Polysaccharides
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
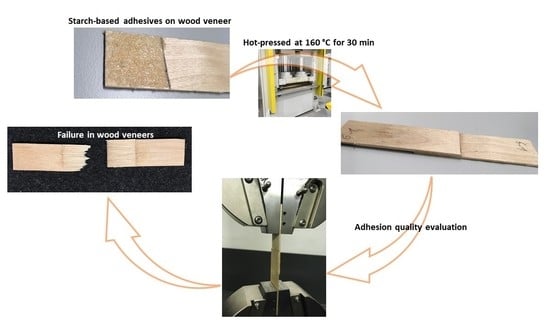
2.2. Adhesive Preparation
2.3. Differential Scanning Calorimetry (DSC) Analysis
2.4. Thermogravimetric Analysis
2.5. Rheological Performance
2.6. Shear Strength Test
2.7. Statistical Analysis
3. Results and Discussion
3.1. Thermal Behavior of the Adhesives
3.2. Rheological Performance
3.3. Tensile Shear Strength
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wood Adhesives and Binders Market Size By Product (Urea-Formaldehyde [UF], Melamine-Urea-Formaldehyde [MUF], Phenol-Formaldehyde [PF], Isocyanates [MDI, TDI, HDI], Soy-Based), By Application (Cabinets, Flooring & Plywood, Furniture & Subcomponents, Windows & Doors), Industry Analysis Report, Regional Outlook, Growth Potential, Price Trends, Competitive Market Share & Forecast, 2016–2024. Available online: https://www.gminsights.com/industry-analysis/wood-adhesives-and-binders-market?gclid=EAIaIQobChMIz6GB2vO27gIVsgV7Ch2mFQpEEAAYASAAEgI5wvD_BwE (accessed on 12 November 2021).
- Cui, S.; Luo, X.; Li, Y. Synthesis and properties of polyurethane wood adhesives derived from crude glycerol-based polyols. Int. J. Adhes. Adhes. 2017, 79, 67–72. [Google Scholar] [CrossRef]
- Hemmilä, V.; Adamopoulos, S.; Karlsson, O.; Kumar, A. Development of sustainable bio-adhesives for engineered wood panels—A Review. RSC Adv. 2017, 7, 38604–38630. [Google Scholar] [CrossRef]
- Gadhave, R.V.; Kasbe, P.S.; Mahanwar, P.A.; Gadekar, P.T. Synthesis and characterization of lignin-polyurethane based wood adhesive. Int. J. Adhes. Adhes. 2019, 95, 102427. [Google Scholar] [CrossRef]
- Cheng, L.; Guo, H.; Gu, Z.; Li, Z.; Hong, Y. Effect of compound emulsifiers on properties of wood adhesive with high starch content. Int. J. Adhes. Adhes. 2017, 72, 92–97. [Google Scholar] [CrossRef]
- Jasiunas, L.; Peck, G.; Bridziuviene, D.; Miknius, L. Mechanical, thermal properties and stability of high renewable content liquefied residual biomass derived bio-polyurethane wood adhesives. Int. J. Adhes. Adhes. 2020, 101, 102618. [Google Scholar] [CrossRef]
- Abraham, T.W.; Carter, J.A.; Malsam, J.; Zlatanic, A.B. Oligomeric Polyols from Palm Based Oils and Polyurethane Compositions Made. Therefrom. Patent Application No. WO2007123637, 1 November 2007. [Google Scholar]
- Roh, Y.; Kumar, R.; Zhao, C.L.; Kaczan, D.; Smiecinski, T.M. BASF, Polyol Formed from an Epoxidized. Oil. Patent Application No. WO2009138411, 19 November 2009. [Google Scholar]
- Shah, A.M.; Shah, T.M. Process for Production Polyols and Polyols for Polyurethane. U.S. Patent 6258869, 10 July 2001. [Google Scholar]
- Keyur, P.S.; Sujata, S.K.; Natvar, K.P.; Animesh, K.R. Castor Oil based polyurethane adhesives for wood-to-wood bonding. Int. J. Adhes. Adhes. 2003, 23, 269–275. [Google Scholar]
- Kong, X.H.; Liu, G.G.; Curtis, J.M. Characterization of canola oil based polyurethane wood adhesives. Int. J. Adhes. Adhes. 2011, 31, 559–564. [Google Scholar] [CrossRef]
- De Menezes, A.J.; Pasquini, D.; Curvelo, A.A.; Gandini, S. Novel thermoplastic materials based on the outer-shell oxypropylation of corn starch granules. Biomacromolecules 2007, 8, 2047–2050. [Google Scholar] [CrossRef]
- Desai, S.D.; Patel, J.V.; Sinha, V.K.; Sinha, V.K. Polyurethane adhesive system from biomaterial-based polyol for bonding wood. Int. J. Adhes. Adhes. 2003, 23, 393–399. [Google Scholar] [CrossRef]
- Briones, R.; Serrano, L.; Younes, R.B.; Mondragon, I.; Labidi, J. Polyol production by chemical modification of date seeds. Ind Crop. Prod. 2011, 34, 1035–1040. [Google Scholar] [CrossRef]
- Hosseinpourpia, R.; Echart, A.S.; Adamopoulos, S.; Gabilondo, N.; Eceiza, A. Modification of pea starch and dextrin polymers with isocyanate functional groups. Polymers 2018, 10, 939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, X.; Cheng, L.; Gu, Z.; Hong, Y.; Li, Z.; Li, C. Effects of heat pretreatment of starch on graft copolymerization reaction and performance of resulting starch-based wood adhesive. Int. J. Biol. Macromol. 2017, 96, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Valodkar, M.; Thakore, S. Isocyanate crosslinked reactive starch nanoparticles for thermo-responsive conducting applications. Carbohydr. Res. 2010, 345, 2354–2360. [Google Scholar] [CrossRef] [PubMed]
- Santayanon, R.; Wootthikanokkhan, J. Modification of cassava starch by using propionic anhydride and properties of the starch-blended polyester polyurethane. Carbohydr. Polym. 2003, 51, 17–24. [Google Scholar] [CrossRef]
- Desai, S.D.; Emanuel, A.L.; Sinha, V.k. Biomaterial Based Polyurethane Adhesive for Bonding Rubber and Wood Joints. J. Polym. Res. 2003, 10, 275–281. [Google Scholar] [CrossRef]
- Qiao, Z.; Gu, J.; Lv, S.; Cao, J.; Tan, H.; Zhang, Y. Preparation and properties of isocyanate prepolymer/corn starch adhesive. J. Adhes. Sci. Technol. 2015, 29, 1368–1381. [Google Scholar] [CrossRef]
- Tan, H.; Zhang, Y.; Weng, X. Preparation of the Plywood Using Starch-based Adhesives Modified with blocked isocyanates. Procedia Eng. 2011, 15, 1171–1175. [Google Scholar] [CrossRef] [Green Version]
- Hosseinpourpia, R.; Adamopoulos, S.; Echart, A.S.; Eceiza, A. Polyurethane films prepared with isophorone diisocyanate functionalized wheat starch. Eur. Polym. J. 2021, 161, 110826. [Google Scholar] [CrossRef]
- Hosseinpourpia, R.; Adamopoulos, S.; Parsland, C. Utilization of different tall oils for improving the water resistance of cellulosic fibers. J. Appl. Polym. Sci. 2019, 136, 47303. [Google Scholar] [CrossRef]
- Hosseinpourpia, R.; Adamopoulos, S.; Mai, C. Tensile strength of handsheets from recovered fibers treated with N-Methylol melamine and 1,3-dimethylol-4,5-dihydroxyethyleneurea. J. Appl. Polym. Sci. 2015, 132, 41290. [Google Scholar] [CrossRef]
- Papadopoulos, E.; Ginic-Markovic, M.; Stephen Clarke, S. A thermal and rheological investigation during the complex cure of a two-component thermoset polyurethane. J. Appl Polym. Sci. 2009, 114, 3802–3810. [Google Scholar] [CrossRef]
- Sonnenschein, M.F. Polyurethanes: Science, Technology, Markets, and Trends; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014. [Google Scholar] [CrossRef]
- Gruke, T. New Advanced in Polymeric MDI Variants, EUROCOAT 2002, Barcelona. Available online: http://docplayer.net/20924200-New-advances-in-polymeric-mdi-variants.html (accessed on 17 November 2021).
- Lu, Y.; Tighzert, L.; Berzin, F.; Rondot, S. Innovative plasticized starch films modified with waterborne polyurethane from renewable resources. Carbohydr. Polym. 2005, 61, 174–182. [Google Scholar] [CrossRef]
- Calvo-Correas, T.; Mosiewicki, M.; Corcuera, M.; Eceiza, A.; Aranguren, M. Linseed oil-based polyurethane rigid foams: Synthesis and characterization. J. Renew. Mater. 2015, 3, 3–13. [Google Scholar] [CrossRef]
- Javaid, M.A.; Rizwan, M.; Khera, R.A.; Zia, K.M.; Saito, K.; Zuber, M.; Iqbal, J.; Langer, P. Thermal degradation behavior and X-ray diffraction studies of chitosan based polyurethane bio-nanocomposites using different diisocyanates. Int. J. Biol. Macromol. 2018, 117, 762–772. [Google Scholar] [CrossRef] [PubMed]
- Levchik, S.; Weil, E. Thermal decomposition, combustion and fire-retardancy of polyurethanes—A review of the recent literature. Polym. Int. 2004, 53, 1585–1610. [Google Scholar] [CrossRef]
- Solankia, A.; Mehtaa, J.; Thakore, S. Structure–property relationships and biocompatibility of carbohydrate crosslinked polyurethanes. Carbohydr. Polym. 2014, 110, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Hejna, A.; Kirpluks, M.; Kosmela, P.; Cabulis, U.; Haponiuk, J.; Piszczyk, Ł. The influence of crude glycerol and castor oil based polyol on the structure and performance of rigid polyurethane-polyisocyanurate foams. Ind. Crops Prod. 2017, 95, 113–125. [Google Scholar] [CrossRef]
- Wang, Z.; Gu, Z.; Hong, Y.; Cheng, L.; Li, Z. Bonding strength and water resistance of starch-based wood adhesive improved by silica nanoparticles. Carbohydr. Polym. 2011, 86, 72–76. [Google Scholar] [CrossRef]
- Marino, R.; Giovando, S.; Gabriele, D. Effect of tannin addition on the rheological properties of starch-based adhesives. Appl. Rheol. 2014, 24, 46138. [Google Scholar]
- Zhang, Y.; Ding, L.; Gu, J.; Tan, H.; Zhu, L. Preparation and properties of a starch-based wood adhesive with high bonding strength and water resistance. Carbohydr. Polym. 2015, 115, 32–37. [Google Scholar] [CrossRef]





| Adhesive | NS (wt. %) | Polyol (wt. %) | MS (wt. %) | pMDI (wt. %) |
|---|---|---|---|---|
| NS-PD-pMDI | 35 | 13 | - | 52 |
| NS-PD-MS1-pMDI | 27 | 12 | 21 | 40 |
| NS-PD-MS2-pMDI | 25 | 10 | 29 | 36 |
| NS-Gly-pMDI | 35 | 11 | - | 54 |
| NS-Gly-MS1-pMDI | 28 | 9 | 22 | 41 |
| NS-Gly-MS2-pMDI | 26 | 8 | 30 | 36 |
| Adhesive | Tmax °C | ∆H (J g−1) |
|---|---|---|
| NS-PD-pMDI | 85.7 | 91.5 |
| NS-PD-MS1-pMDI | 68.3 | 27.7 |
| NS-PD-MS2-pMDI | 67.3 | 23.5 |
| NS-Gly-pMDI | 127.2 | 83.1 |
| NS-Gly-MS1-pMDI | 114.5 | 74.7 |
| NS-Gly-MS2-Gly | 106.7 | 50.6 |
| Adhesive | Tmax1 | Tmax2 | Tmax3 | Tmax4 | RW |
|---|---|---|---|---|---|
| (°C) | (°C) | (°C) | (°C) | (%) | |
| NS-PD-pMDI | 60.2 | 157.2 | 311.8 | 442.0 | 21.7 |
| NS-PD-MS1-pMDI | 60.6 | 156.4 | 325.5 | 438.7 | 17.3 |
| NS-PD-MS2-pMDI | 58.4 | 156.1 | 326.9 | 438.1 | 16.4 |
| NS-Gly-pMDI | 64.7 | 215.9 | 315.6 | 443.0 | 20.7 |
| NS-Gly-MS1-pMDI | 62.0 | 216.9 | 325.4 | 427.6 | 17.8 |
| NS-Gly-MS2-pMDI | 64.5 | 217.6 | 326.1 | 439.5 | 17.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hosseinpourpia, R.; Eceiza, A.; Adamopoulos, S. Polyurethane Wood Adhesives Prepared from Modified Polysaccharides. Polymers 2022, 14, 539. https://doi.org/10.3390/polym14030539
Hosseinpourpia R, Eceiza A, Adamopoulos S. Polyurethane Wood Adhesives Prepared from Modified Polysaccharides. Polymers. 2022; 14(3):539. https://doi.org/10.3390/polym14030539
Chicago/Turabian StyleHosseinpourpia, Reza, Arantxa Eceiza, and Stergios Adamopoulos. 2022. "Polyurethane Wood Adhesives Prepared from Modified Polysaccharides" Polymers 14, no. 3: 539. https://doi.org/10.3390/polym14030539