An Attempt to Optimize Supercritical CO2 Polyaniline-Polycaprolactone Foaming Processes to Produce Tissue Engineering Scaffolds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
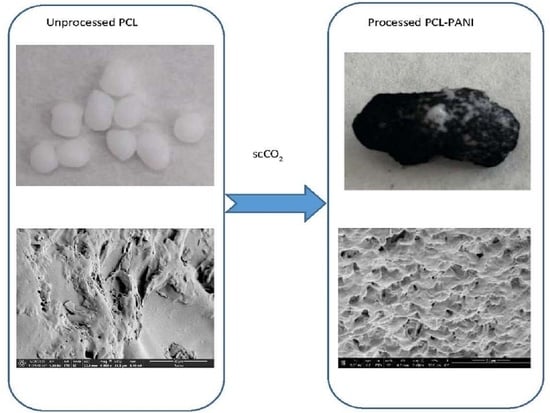
2.2. Supercritical Foaming Process
2.3. Scanning Electron Microscopy
2.4. Porosity Estimation
2.5. Estimating the Expansion Factor
2.6. Estimating the Amount of PANI Incorporated into the Scaffold
2.7. Biodegradability
2.8. Compression Test
2.9. Electrical Properties
3. Results and Discussion
Foaming Process
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Toh, H.W.; Toong, D.W.Y.; Ng, J.C.K.; Ow, V.; Lu, S.; Tan, L.P.; Wong, P.E.H.; Venkatraman, S.; Huang, Y.; Ang, H.Y. Polymer blends and polymer composites for cardiovascular implants. Eur. Polym. J. 2021, 146, 110249. [Google Scholar] [CrossRef]
- Krishna, E.S.; Suresh, G. Bioactive Titanium-Hydroxyapatite Composites by Powder Metallurgy Route. Bionterface Res. Appl. Chem. 2022, 12, 5375–5383. [Google Scholar] [CrossRef]
- Des-champs, A.A.; Claase, M.B.; Sleijster, W.J.; de Bruijn, J.D.; Grijpma, D.W.; Feijen, J. Design of seg-mented poly (ether ester) materials and structures for the tissue engineering of bone. J. Control. Release 2002, 78, 175–186. [Google Scholar] [CrossRef]
- Liao, S.S.; Guan, K.; Cui, F.Z.; Shi, S.S.; Sun, T.S. Lumbar spinal fusion with a mineralized collagen matrix and rhBMP-2 in a rabbit model. Spine 2003, 28, 1954–1960. [Google Scholar] [CrossRef] [PubMed]
- Lam, M.T.; Wu, J.C. Biomaterial applications in cardiovascular tissue repair and regeneration. Expert Rev. Cardiovasc. Ther. 2012, 10, 1039–1049. [Google Scholar] [CrossRef] [PubMed]
- Cosson, M.; Debodinance, P.; Boukerrou, M.; Chauvet, M.P.; Lobry, P.; Crépin, G.; Ego, A. Mechanical properties of synthetic implants used in the repair of prolapse and urinary incontinence in women: Which is the ideal material? Int. Urogynecol. J. 2003, 14, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Maitz, M.F. Applications of synthetic polymers in clinical medicine. Biosurf. Biotribol. 2015, 1, 161–176. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Jiang, M.; Gao, Y.; Zheng, Y.; Liu, Z.i.; Zhou, C.; Huang, T.; Gu, X.; Li, A.; Fang, J.; et al. Current status and outlook of biodegradable metals in neuroscience and their potential applications as cerebral vascular stent materials. Bioact. Mater. 2022, 11, 140–153. [Google Scholar] [CrossRef]
- Toong, D.W.Y.; Ng, J.C.K.; Huang, Y.; Wong, P.E.H.; Leo, H.L.; Venkatraman, S.S.; Ang, H.Y. Bioresorbable metals in cardiovascular stents: Material insights and progress. Materialia 2020, 12, 100727. [Google Scholar] [CrossRef]
- Li, G.; Yang, H.; Zheng, Y.; Chen, X.H.; Yang, J.A.; Zhu, D.; Ruan, L.; Takashima, K. Challenges in the use of zinc and its alloys as biodegradable metals: Perspective from biomechanical compatibility. Acta Biomater. 2019, 97, 23–45. [Google Scholar] [CrossRef]
- Han, H.-S.; Loffredo, S.; Jun, I.; Edwards, J.; Kim, Y.-C.; Seok, H.-K.; Witte, F.; Mantovani, D.; Glyn-Jones, S. Current status and outlook on the clinical translation of biodegradable metals. Mater. Today 2019, 23, 57–71. [Google Scholar] [CrossRef]
- Xia, D.; Yang, F.; Zheng, Y.; Liu, Y.; Zhou, Y. Research status of biodegradable metals designed for oral and maxillofacial applications: A review. Bioact. Mater. 2021, 6, 4186–4208. [Google Scholar] [CrossRef]
- Shaoqiong, L.; Chau-Sang, L.; Kun, L.; Feng, W.; Hin, T.S. Marine collagen scaffolds in tissue engineering. Curr. Opin. Biotechnol. 2022, 74, 92–103. [Google Scholar] [CrossRef]
- Sharifi, S.; Khosroshahi, A.Z.; Dizaj, S.M.; Rezaei, Y. Preparation, Physicochemical Assessment and the Antimicrobial Action of Hydroxyapatite–Gelatin/Curcumin Nanofibrous Composites as a Dental Biomaterial. Biomimetics 2022, 7, 4. [Google Scholar] [CrossRef]
- Liu, L.; Ji, X.; Mao, L.; Wang, L.; Chen, K.; Shi, Z.; Ahmed, A.A.Q.; Thomas, S.; Vasilievich, R.V.; Xiao, L.; et al. Hierarchical-structured bacterial cellulose/potato starch tubes as potential small-diameter vascular grafts. Carbohydr. Polym. 2022, 281, 119034. [Google Scholar] [CrossRef]
- Sukpaita, T.; Chirachanchai, S.; Pimkhaokham, A.; Ampornaramveth, R.S. Chitosan-Based Scaffold for Mineralized Tissues Regeneration. Mar. Drugs 2021, 19, 551. [Google Scholar] [CrossRef]
- Wang, S.; Li, R.; Xia, D.; Zhao, X.; Zhu, Y.; Gu, R.; Yoon, J.; Liu, Y. The impact of Zn-doped synthetic polymermaterials on bone regeneration: A systematic review. Stem Cell Res. Ther. 2021, 12, 123. [Google Scholar] [CrossRef]
- Funk, R.H.W.; Monsees, T.; Özkucur, N. Electromagnetic effects—From cell biology to medicine. Prog. Histochem. Cytochem. 2009, 43, 177–264. [Google Scholar] [CrossRef]
- Vasquez-Sancho, F.; Abdollahi, A.; Damjanovic, D.; Catalan, G. Flexoelectricity in Bones. Adv. Mater. 2018, 30, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Nezakati, T.; Seifalian, A.; Tan, A.; Seifalian, A.M. Conductive Polymers: Opportunities and Challenges in Biomedical Applications. Chem. Rev. 2018, 118, 6766–6843. [Google Scholar] [CrossRef]
- Kargirwar, S.R.; Thakare, S.R.; Choudhary, M.D.; Kondawar, S.B.; Dhakate, S.R. Morphology and electrical conductivity of self-doping polyanilines synthesized via self-assembly process. Adv. Mater. Lett. 2011, 2, 397–401. [Google Scholar] [CrossRef]
- Balint, R.; Cassidy, N.J.; Cartmell, S.H. Conductive polymers: Towards a smart biomaterial for tissue engineering. Acta Biomater. 2014, 10, 2341–2353. [Google Scholar] [CrossRef] [PubMed]
- Golbaten-Mofrad, H.; Sahzabi, A.S.; Seyfikar, S.; Salehi, M.H.; Goodarzi, V.; Wurm, F.R.; Jafari, S.H. Facile template preparation of novel electroactive scaffold composed of polypyrrole-coated poly(glycerol-sebacate-urethane) for tissue engineering applications. Eur. Polym. J. 2021, 159, 110749. [Google Scholar] [CrossRef]
- Rajzer, I.; Rom, M.; Menaszek, E.; Fabia, J.; Kwiatkowski, R. Conductive Polyaniline Patterns on Electrospun Polycaprolactone/Hydroxyapatite Scaffolds for Bone Tissue Engineering. Materials 2021, 14, 4837. [Google Scholar] [CrossRef] [PubMed]
- Rivers, T.J.; Hudson, T.W.; Schmidt, C.E. Synthesis of a novel, biodegradable electrically conducting polymer for biomedical applications. Adv. Funct. Mater 2002, 33, 7. [Google Scholar] [CrossRef]
- Borriello, A.; Schiavo, V.G.L.; Alvarez-Perez, M.A.; Ambrosio, L. Optimizing PANi doped electroactive substrates as patches for the regeneration of cardiac muscle. J. Mater. Sci. Mater. Med. 2011, 22, 1053–1062. [Google Scholar] [CrossRef]
- Gil-Castell, O.; Ontoria-Oviedo, I.; Badia, J.D.; Amaro-Prellezo, E.; Sepúlveda, P.; Ribes-Greus, A. Conductive polycaprolac-tone/gelatin/polyaniline nanofibres as functional scaffolds for cardiac tissue regeneration. React. Funct. Polym. 2022, 170, 105064. [Google Scholar] [CrossRef]
- Beregoi, M.; Busuioc, C.; Evanghelidis, A.; Matei, E.; Iordache, F.; Radu, M.; Dinischiotu, A.; Enculescu, I. Electrochromic properties of polyaniline-coated fiber webs for tissue engineering applications. Int. J. Pharm. 2016, 510, 465–473. [Google Scholar] [CrossRef]
- Gizdavic-Nikolaidis, M.; Pupe, J.M.; Silva, L.P.; Stanisavljev, D.; Svirskis, D.; Swift, S. Composition tuning of scalable antibacterial polyaniline/chitosan composites through rapid enhanced microwave synthesis. Mater. Chem. Phys. 2022, 278, 125676. [Google Scholar] [CrossRef]
- Cullen, D.K.; Patel, A.R.; Doorish, J.F.; Smith, D.H.; Pfister, B.J. Developing a tissue engineered neural-electrical relay using encapsulated neuronal constructs on conducting polymer fibers. J. Neural. Eng. 2008, 5, 374–384. [Google Scholar] [CrossRef]
- Ravi, M.; Paramesh, V.; Kaviya, S.R.; Anuradha, E.; Solomon, F.D.P. 3D cell culture systems: Advantages and applications. J. Cell. Physiol. 2015, 230, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Jie, W.; Song, F.; Li, X.; Li, W.; Wang, R.; Jiang, Y.; Zhao, L.; Fan, Z.; Wang, J.; Liu, B. Enhancing the proliferation of MC3T3-E1 cells on casein phosphopeptide-biofunctionalized 3D reduced-graphene oxide/polypyrrole scaffolds. RSC Adv. 2017, 7, 34415–34424. [Google Scholar] [CrossRef] [Green Version]
- Velasco, D.; Benito, L.; Fernández-Gutiérrez, M.; Román, J.S.; Elvira, C. Preparation in supercritical CO2 of porous poly(methyl methacrylate)-poly(l-lactic acid) (PMMA-PLA) scaffolds incorporating ibuprofen. J. Supercrit. Fluids 2010, 54, 335–341. [Google Scholar] [CrossRef]
- Mooney, D.J.; Baldwin, D.F.; Suh, N.P.; Vacanti, J.P.; Langer, R. Novel approach to fabricate porous sponges of poly(D,L-lactic-co-glycolic acid) without the use of organic solvents. Biomaterials 1996, 17, 1417–1422. [Google Scholar] [CrossRef]
- Murphy, W.L.; Dennis, R.G.; Kileny, J.L.; Mooney, D.J. Salt fusion: An approach to improve pore interconnectivity within tissue engineering scaffolds. Tissue Eng. 2002, 8, 43–52. [Google Scholar] [CrossRef]
- Ma, P.X.; Choi, J.W. Biodegradable polymer scaffolds with well-defined interconnected spherical pore network. Tissue Eng. 2001, 7, 23–33. [Google Scholar] [CrossRef] [Green Version]
- Alegret, N.; Dominguez-Alfaro, A.; Mecerreyes, D. 3D Scaffolds Based on Conductive Polymers for Biomedical Applications. Biomacromolecules 2019, 20, 73–89. [Google Scholar] [CrossRef]
- García-Casas, I.; Montes, A.; Valor, D.; Pereyra, C.; de la Ossa, E.J.M. Foaming of polycaprolactone and its impregnation with quercetin using supercritical CO2. Polymers 2019, 11, 1390. [Google Scholar] [CrossRef] [Green Version]
- Cabezas, L.I.; Fernández, V.; Mazarro, R.; Gracia, I.; de Lucas, A.; Cabezas, J.F.R. Production of biodegradable porous scaffolds impregnated with indomethacin in supercritical CO2. J. Supercrit. Fluids 2012, 63, 155–160. [Google Scholar] [CrossRef]
- Fanovich, M.A.; Ivanovic, J.; Misic, D.; Alvarez, M.V.; Jaeger, P.; Zizovic, I.; Eggers, R. Development of polycaprolactone scaffold with antibacterial activity by an integrated supercritical extraction and impregnation process. J. Supercrit. Fluids 2013, 78, 42–53. [Google Scholar] [CrossRef]
- Campardelli, R.; Franco, P.; Reverchon, E.; de Marco, I. Polycaprolactone/nimesulide patches obtained by a one-step supercritical foaming + impregnation process. J. Supercrit. Fluids 2019, 146, 47–54. [Google Scholar] [CrossRef]
- Godoy-Gallardo, M.; Portolés-Gil, N.; López-Periago, A.M.; Domingo, C.; Hosta-Rigau, L. Multi-layered polydopamine coatings for the immobilization of growth factors onto highly-interconnected and bimodal PCL/HA-based scaffolds. Mater. Sci. Eng. 2020, 117, 111245. [Google Scholar] [CrossRef] [PubMed]
- Santos-Rosales, V.; Magariños, B.; Alvarez-Lorenzo, C.; García-González, C.A. Combined sterilization and fabrication of drug-loaded scaffolds using supercritical CO2 technology. Int. J. Pharm. 2022, 612, 121362. [Google Scholar] [CrossRef] [PubMed]
- Xiang, P.; Gou, L.; Zou, Y.; Chen, B.; Bi, S.; Chen, X.; Yu, P. A facile strategy for preparation of strong tough poly(lactic acid) foam with a unique microfibrillated bimodal micro/nano cellular structure. Int. J. Biol. Macromol. 2022, 199, 264–274. [Google Scholar] [CrossRef]
- Santos, L.F.; Correia, I.J.; Silva, A.S.; Mano, J.F. Biomaterials for drug delivery patches. Eur. J. Pharm. Sci. 2018, 118, 49–66. [Google Scholar] [CrossRef]
- Labet, M.; Thielemans, W. Synthesis of polycaprolactone: A review. Chem. Soc. Rev. 2009, 38, 3484–3504. [Google Scholar] [CrossRef]
- Moghadam, M.Z.; Hassanajili, S.; Esmaeilzadeh, F.; Ayatollahi, M.; Ahmadi, M. Formation of porous HPCL/LPCL/HA scaffolds with supercritical CO2 gas foaming method. J. Mech. Behav. Biomed. Mater. 2017, 69, 115–127. [Google Scholar] [CrossRef]
- Mou, Z.L.; Zhao, L.J.; Zhang, Q.A.; Zhang, J.; Zhang, Z.Q. Preparation of porous PLGA/HA/collagen scaffolds with supercritical CO2 and application in osteoblast cell culture. J. Supercrit. Fluids 2011, 58, 398–406. [Google Scholar] [CrossRef]
- White, L.J.; Hutter, V.; Tai, H.; Howdle, S.M.; Shakesheff, K.M. The effect of processing variables on morphological and mechanical properties of supercritical CO2 foamed scaffolds for tissue engineering. Acta Biomater. 2012, 8, 61–71. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Liu, Q.; Xin, X.; Guan, Y.; Yao, S. Pore formation of poly(ε-caprolactone) scaffolds with melting point reduction in supercritical CO2 foaming. J. Supercrit. Fluids 2016, 117, 279–288. [Google Scholar] [CrossRef]
- Byrne, D.P.; Lacroix, D.; Planell, J.A.; Kelly, D.J.; Prendergast, P.J. Simulation of tissue differentiation in a scaffold as a function of porosity, Young’s modulus and dissolution rate: Application of mechanobiological models in tissue engineering. Biomaterials 2007, 28, 5544–5554. [Google Scholar] [CrossRef] [PubMed]









| Runs | P (bar) | T (°C) | Dr (bar/min) | Ratio PCL:PANI |
|---|---|---|---|---|
| 1 | 300 | 40 | 20 | 1:1 |
| 2 | 300 | 40 | 20 | 5:1 |
| 3 | 300 | 40 | 20 | 10:1 |
| 4 | 100 | 40 | 20 | 20:1 |
| 5 | 300 | 40 | 50 | 5:1 |
| 6 | 300 | 70 | 50 | 5:1 |
| 7 | 300 | 70 | 20 | 5:1 |
| 8 | 100 | 70 | 20 | 5:1 |
| 9 | 100 | 70 | 50 | 5:1 |
| 10 | 300 | 40 | 20 | 5:1 |
| Run | P (bar) | T (°C) | Dr (bar/min) | P 1 (%) | EF 2 (%) | PL 3 (%) | B 4 (%) | I 5 (Ω) | E 6 (MPa) | PS 7 (MPa) |
|---|---|---|---|---|---|---|---|---|---|---|
| 5 | 300 | 40 | 50 | 33 | 2.68 | 9.19 | 1.64 | 1.90·108 | 58.24 | 34.51 |
| 6 | 300 | 70 | 50 | 31 | 4.88 | 28.50 | 2.35 | 2.03·108 | - | 8.47 |
| 7 | 300 | 70 | 20 | 50 | 4.30 | 4.46 | 1.87 | 4.67·108 | 2.56 | 8.02 |
| 8 | 100 | 70 | 20 | 50 | 4.74 | 22.51 | 5.59 | 2.35·105 | 12.00 | 3.61 |
| 9 | 100 | 70 | 50 | 50 | 4.29 | 7.93 | 9.54 | 1.99·106 | 2.41 | 1.76 |
| 10 | 300 | 40 | 20 | 10 | 2.67 | 22.48 | 0.00 | 4.85·108 | 15.56 | 10.41 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montes, A.; Valor, D.; Delgado, L.; Pereyra, C.; Martínez de la Ossa, E. An Attempt to Optimize Supercritical CO2 Polyaniline-Polycaprolactone Foaming Processes to Produce Tissue Engineering Scaffolds. Polymers 2022, 14, 488. https://doi.org/10.3390/polym14030488
Montes A, Valor D, Delgado L, Pereyra C, Martínez de la Ossa E. An Attempt to Optimize Supercritical CO2 Polyaniline-Polycaprolactone Foaming Processes to Produce Tissue Engineering Scaffolds. Polymers. 2022; 14(3):488. https://doi.org/10.3390/polym14030488
Chicago/Turabian StyleMontes, Antonio, Diego Valor, Laura Delgado, Clara Pereyra, and Enrique Martínez de la Ossa. 2022. "An Attempt to Optimize Supercritical CO2 Polyaniline-Polycaprolactone Foaming Processes to Produce Tissue Engineering Scaffolds" Polymers 14, no. 3: 488. https://doi.org/10.3390/polym14030488