Improvement of High-Throughput Experimentation Using Synthesis Robots by the Implementation of Tailor-Made Sensors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Methods
2.2. Polymer Synthesis
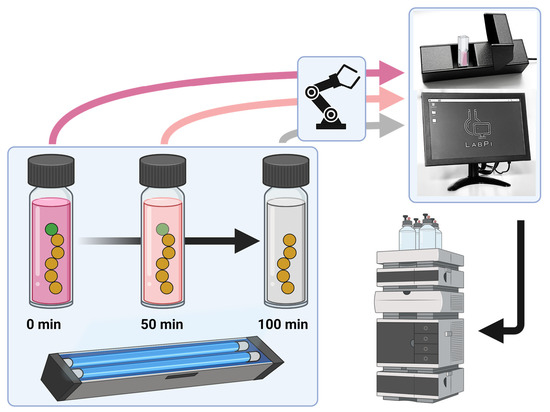
2.3. UV-Induced RAFT End-Group Degradation
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bosman, A.W.; Heumann, A.; Klaerner, G.; Benoit, D.; Fréchet, J.M.J.; Hawker, C.J. High-Throughput Synthesis of Nanoscale Materials: Structural Optimization of Functionalized One-Step Star Polymers. J. Am. Chem. Soc. 2001, 123, 6461–6462. [Google Scholar] [CrossRef] [PubMed]
- Voorhaar, L.; Hoogenboom, R. One-Pot Synthesis of Charged Amphiphilic Diblock and Triblock Copolymers Via High-Throughput Cu(0)-Mediated Polymerization. Polymers 2017, 9, 320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoogenboom, R.; Fijten, M.W.M.; Abeln, C.H.; Schubert, U.S. High-Throughput Investigation of Polymerization Kinetics by Online Monitoring of GPC and GC. Macromol. Rapid Commun. 2004, 25, 237–242. [Google Scholar] [CrossRef]
- Fijten, M.W.M.; Paulus, R.M.; Schubert, U.S. Systematic parallel investigation of RAFT polymerizations for eight different (meth)acrylates: A basis for the designed synthesis of block and random copolymers. J. Polym. Sci. Part A Polym. Chem. 2005, 43, 3831–3839. [Google Scholar] [CrossRef]
- Zhang, H.H.; Marin, V.V.; Fijten, M.W.M.; Schubert, U.S. High-throughput experimentation in atom transfer radical polymerization: A general approach toward a directed design and understanding of optimal catalytic systems. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 1876–1885. [Google Scholar] [CrossRef]
- Siegwart, D.J.; Whitehead, K.A.; Nuhn, L.; Sahay, G.; Cheng, H.; Jiang, S.; Ma, M.; Lytton-Jean, A.; Vegas, A.; Fenton, P.; et al. Combinatorial synthesis of chemically diverse core-shell nanoparticles for intracellular delivery. Proc. Natl. Acad. Sci. USA 2011, 108, 12996–13001. [Google Scholar] [CrossRef] [Green Version]
- LaRosa, C.; Stura, E.; Eggenhoffner, R.; Nicolini, C. Optimization of Optical Properties of Polycarbonate Film with Thiol Gold-Nanoparticles. Materials 2009, 2, 1193–1204. [Google Scholar] [CrossRef]
- Sagmeister, P.; Lebl, R.; Castillo, I.; Rehrl, J.; Kruisz, J.; Sipek, M.; Horn, M.; Sacher, S.; Cantillo, D.; Williams, J.D.; et al. Advanced Real-Time Process Analytics for Multistep Synthesis in Continuous Flow. Angew. Chem. Int. Ed. 2021, 60, 8139–8148. [Google Scholar] [CrossRef] [PubMed]
- Salley, D.; Keenan, G.; Grizou, J.; Sharma, A.; Martín, S.; Cronin, L. A nanomaterials discovery robot for the Darwinian evolution of shape programmable gold nanoparticles. Nat. Commun. 2020, 11, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Höpfner, J.; Ratzsch, K.-F.; Botha, C.; Wilhelm, M. Medium Resolution 1 H-NMR at 62 MHz as a New Chemically Sensitive Online Detector for Size-Exclusion Chromatography (SEC-NMR). Macromol. Rapid Commun. 2018, 39, e1700766. [Google Scholar] [CrossRef] [PubMed]
- Guidi, M.; Seeberger, P.H.; Gilmore, K. How to approach flow chemistry. Chem. Soc. Rev. 2020, 49, 8910–8932. [Google Scholar] [CrossRef]
- Andraos, S.; Goy, M.; Albert, B.B.; Kussmann, M.; Thorstensen, E.B.; O’Sullivan, J.M. Robotic automation of a UHPLC/MS-MS method profiling one-carbon metabolites, amino acids, and precursors in plasma. Anal. Biochem. 2020, 592, 113558. [Google Scholar] [CrossRef]
- Wejner, M.; Wilke, T. Low Cost—High Tech: The Digital Measurement System LabPi. CHEMKON 2019, 26, 294–300. [Google Scholar] [CrossRef]
- Wejner, M.; Wilke, T. LabPi: A Digital Measuring Station for STEM Education 4.0. J. Chem. Educ. 2022. [Google Scholar] [CrossRef]
- Christ, H.-A.; Bourgat, Y.; Menzel, H. Optimization of Critical Parameters for Carbodiimide Mediated Production of Highly Modified Chitosan. Polymer 2021, 13, 2702. [Google Scholar] [CrossRef]
- Wejner, M.; Wilke, T. Air quality: An inexpensive CO2 indicator for the classroom with LabPi. CHEMKON 2021. [Google Scholar] [CrossRef]
- Petersen, M.; Worliczek, P.; Max, J.B.; Nabiyan, A.; Wejner, M.; Eichhorn, J.; Streb, C.; Schacher, F.H.; Wilke, T. Hydrogen Evolution Reaction with Sunlight for School Chemistry Education. World J. Chem. Educ. 2021, 9, 190–196. [Google Scholar] [CrossRef]
- Manuel Wejner, T.W. LabPi—A Powerful, Digital Low-Cost Measuring Station for STEM Education. In Proceedings of the 9th Edition of the New Perspectives in Science Education, Florence, Italy, 19–20 March 2020; pp. 54–58. [Google Scholar]
- Chiefari, J.; Chong, Y.K.; Ercole, F.; Krstina, J.; Jeffery, J.; Le, T.P.T.; Mayadunne, R.T.A.; Meijs, G.F.; Moad, C.L.; Moad, G.; et al. Living Free-Radical Polymerization by Reversible Addition−Fragmentation Chain Transfer: The RAFT Process. Macromolecules 1998, 31, 5559–5562. [Google Scholar] [CrossRef]
- Cockram, A.A.; Bradley, R.D.; Lynch, S.A.; Fleming, P.C.D.; Williams, N.S.J.; Murray, M.W.; Emmett, S.N.; Armes, S.P. Optimization of the high-throughput synthesis of multiblock copolymer nanoparticles in aqueous media via polymerization-induced self-assembly. React. Chem. Eng. 2018, 3, 645–657. [Google Scholar] [CrossRef] [Green Version]
- Guerrero-Sanchez, C.; Keddie, D.J.; Saubern, S.; Chiefari, J. Automated Parallel Freeze–Evacuate–Thaw Degassing Method for Oxygen-Sensitive Reactions: RAFT Polymerization. ACS Comb. Sci. 2012, 14, 389–394. [Google Scholar] [CrossRef]
- Aerts, A.; Lewis, R.; Zhou, Y.; Malic, N.; Moad, G.; Postma, A. Light-Induced RAFT Single Unit Monomer Insertion in Aqueous Solution-Toward Sequence-Controlled Polymers. Macromol. Rapid Commun. 2018, 39, e1800240. [Google Scholar] [CrossRef]
- Ng, G.; Yeow, J.; Chapman, R.; Isahak, N.; Wolvetang, E.; Cooper-White, J.J.; Boyer, C. Pushing the Limits of High Throughput PET-RAFT Polymerization. Macromolecules 2018, 51, 7600–7607. [Google Scholar] [CrossRef]
- Moad, G.; Rizzardo, E.; Thang, S. Living Radical Polymerization by the RAFT Process—A Third Update. Aust. J. Chem. 2012, 65, 985–1076. [Google Scholar] [CrossRef]
- Moad, G.; Rizzardo, E.; Thang, S.H. RAFT Polymerization and Some of its Applications. Chem.-Asian J. 2013, 8, 1634–1644. [Google Scholar] [CrossRef]
- Inglis, A.J.; Sinnwell, S.; Davis, T.; Barner-Kowollik, C.; Stenzel, M.H. Reversible Addition Fragmentation Chain Transfer (RAFT) and Hetero-Diels−Alder Chemistry as a Convenient Conjugation Tool for Access to Complex Macromolecular Designs. Macromolecules 2008, 41, 4120–4126. [Google Scholar] [CrossRef]
- Bode, S.; Enke, M.; Görls, H.; Hoeppener, S.; Weberskirch, R.; Hager, M.D.; Schubert, U.S. Blocked isocyanates: An efficient tool for post-polymerization modification of polymers. Polym. Chem. 2014, 5, 2574–2582. [Google Scholar] [CrossRef]
- Whittaker, M.; Goh, Y.-K.; Gemici, H.; Legge, T.M.; Perrier, S.; Monteiro, M. Synthesis of Monocyclic and Linear Polystyrene Using the Reversible Coupling/Cleavage of Thiol/Disulfide Groups. Macromolecules 2006, 39, 9028–9034. [Google Scholar] [CrossRef]
- De Brouwer, H.; Schellekens, M.A.J.; Klumperman, B.; Monteiro, M.; German, A.L. Controlled radical copolymerization of styrene and maleic anhydride and the synthesis of novel polyolefin-based block copolymers by reversible addition-fragmentation chain-transfer (RAFT) polymerization. J. Polym. Sci. Part A Polym. Chem. 2000, 38, 3596–3603. [Google Scholar] [CrossRef]
- Moad, G.; Chen, M.; Häussler, M.; Postma, A.; Rizzardo, E.; Thang, S.H. Functional polymers for optoelectronic applications by RAFT polymerization. Polym. Chem. 2011, 2, 492–519. [Google Scholar] [CrossRef]
- Ramesh, K.; Mishra, A.K.; Kim, J.K.; Jeong, Y.T.; Gal, Y.-S.; Lim, K.T. Preparation of Doxorubicin-Loaded Amphiphilic Poly(D,L-Lactide-Co-Glycolide)-b-Poly(N-Acryloylmorpholine) AB2 Miktoarm Star Block Copolymers for Anticancer Drug Delivery. Materials 2020, 13, 3713. [Google Scholar] [CrossRef]
- Thomas, M.; Lu, J.J.; Zhang, C.; Chen, J.; Klibanov, A.M. Identification of Novel Superior Polycationic Vectors for Gene Delivery by High-throughput Synthesis and Screening of a Combinatorial Library. Pharm. Res. 2007, 24, 1564–1571. [Google Scholar] [CrossRef] [PubMed]
- LabPi: Eigenbau. Available online: https://labpi.de/eigenbau.php (accessed on 3 November 2021).
- Schuett, T.; Kimmig, J.; Zechel, S.; Schubert, U.S. Automated Polymer Purification Using Dialysis. Polymer 2020, 12, 2095. [Google Scholar] [CrossRef] [PubMed]
- Kimmig, J.; Schuett, T.; Vollrath, A.; Zechel, S.; Schubert, U.S. Prediction of Nanoparticle Sizes for Arbitrary Methacrylates Using Artificial Neuronal Networks. Adv. Sci. 2021, 8, 2102429. [Google Scholar] [CrossRef]
- LabPi COMPare. Available online: www.labpi.de/compare (accessed on 3 November 2021).




| Polymer | Monomer | m(Monomer) (g) | V(DMF) (mL) | m(CPDB) (mg) | m(AIBN) (mg) |
|---|---|---|---|---|---|
| P1 | PEGMEMA | 100 | 100 | 885.4 | 164.2 |
| P2 | MMA | 80 | 400 | 884.3 | 164.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schuett, T.; Wejner, M.; Kimmig, J.; Zechel, S.; Wilke, T.; Schubert, U.S. Improvement of High-Throughput Experimentation Using Synthesis Robots by the Implementation of Tailor-Made Sensors. Polymers 2022, 14, 361. https://doi.org/10.3390/polym14030361
Schuett T, Wejner M, Kimmig J, Zechel S, Wilke T, Schubert US. Improvement of High-Throughput Experimentation Using Synthesis Robots by the Implementation of Tailor-Made Sensors. Polymers. 2022; 14(3):361. https://doi.org/10.3390/polym14030361
Chicago/Turabian StyleSchuett, Timo, Manuel Wejner, Julian Kimmig, Stefan Zechel, Timm Wilke, and Ulrich S. Schubert. 2022. "Improvement of High-Throughput Experimentation Using Synthesis Robots by the Implementation of Tailor-Made Sensors" Polymers 14, no. 3: 361. https://doi.org/10.3390/polym14030361