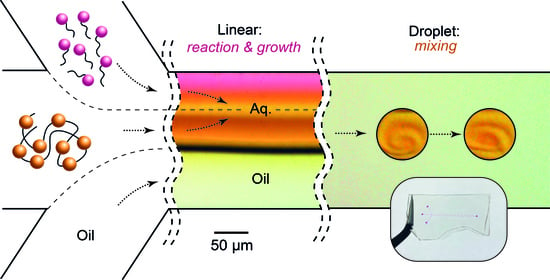
Tuning Properties of Polyelectrolyte-Surfactant Associates in Two-Phase Microfluidic Flows
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Solutions
2.3. Methods
2.4. Device Fabrication
3. Results and Discussion
3.1. Characterization of PDADMAC-SDS Complexes Synthesized in Macroscopic Conditions
3.2. Determining Flow Conditions for Performing PDADMAC-SDS Association in Microfluidic Droplets and Fluid Threads
3.3. Synthesis of PDADMAC-SDS Complexes in the Droplet Mode: Impact of Droplet Size and Microchannel Geometry
3.4. Synthesis of PDADMAC-SDS Complexes in the Thread Mode: Impact of Thread Width and Thread Break-Up
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Langevin, D. Complexation of oppositely charged polyelectrolytes and surfactants in aqueous solutions. A review. Adv. Colloid Interface Sci. 2009, 147–148, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Goddard, E.D.; Goddard, E.D.; Ananthapadmanabhan, K.P. Interactions of Surfactants with Polymers and Proteins; Boca Raton CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Gradzielski, M.; Hoffmann, I. Polyelectrolyte-surfactant complexes (PESCs) composed of oppositely charged components. Curr. Opin. Colloid Interface Sci. 2018, 35, 124–141. [Google Scholar] [CrossRef]
- Wu, D.; Zhu, L.; Li, Y.; Zhang, X.; Xu, S.; Yang, G.; Delair, T. Chitosan-based Colloidal Polyelectrolyte Complexes for Drug Delivery: A Review. Carbohydr. Polym. 2020, 238, 116126. [Google Scholar] [CrossRef] [PubMed]
- Ritacco, H.A. Polyelectrolyte/Surfactant Mixtures: A Pathway to Smart Foams. ACS Omega 2022, 7, 36117–36136. [Google Scholar] [CrossRef] [PubMed]
- Holmberg, K.; Jönsson, B.; Kronberg, B.; Lindman, B. Surfactants and Polymers in Aqueous Solution, 2nd ed.; Wiley: Hoboken, NJ, USA, 2003; p. 545. [Google Scholar]
- Hu, Q.; Li, H.; Wang, L.; Gu, H.; Fan, C. DNA Nanotechnology-Enabled Drug Delivery Systems. Chem. Rev. 2019, 119, 6459–6506. [Google Scholar] [CrossRef] [PubMed]
- Ristroph, K.D.; Prud’Homme, R.K. Hydrophobic ion pairing: Encapsulating small molecules, peptides, and proteins into nanocarriers. Nanoscale Adv. 2019, 1, 4207–4237. [Google Scholar] [CrossRef] [Green Version]
- Klymchenko, A.S.; Liu, F.; Collot, M.; Anton, N. Dye-Loaded Nanoemulsions: Biomimetic Fluorescent Nanocarriers for Bioimaging and Nanomedicine. Adv. Health Mater. 2021, 10, e2001289. [Google Scholar] [CrossRef]
- Shi, C.; Du, G.; Wang, J.; Sun, P.; Chen, T. Polyelectrolyte–Surfactant Mesomorphous Complex Templating: A Versatile Approach for Hierarchically Porous Materials. Langmuir 2020, 36, 1851–1863. [Google Scholar] [CrossRef]
- Wang, W.X.; Deng, S.X.; Shi, C.X.; Sun, P.C.; Chen, T.H. Synthesis of mesoporous silica using anionic surfactant/cationic polyamine as a template. Wuli Huaxue Xuebao/Acta Phys.-Chim. Sin. 2015, 31, 707–714. [Google Scholar]
- Molchanov, V.S.; Shibaev, A.V.; Karamov, E.V.; Larichev, V.F.; Kornilaeva, G.V.; Fedyakina, I.T.; Turgiev, A.S.; Philippova, O.E.; Khokhlov, A.R. Antiseptic Polymer—Surfactant Complexes with Long-Lasting Activity against SARS-CoV. Polymers 2022, 14, 2444. [Google Scholar] [CrossRef]
- Gradzielski, M. Polyelectrolyte-Surfactant Complexes as a Formulation Tool for Drug Delivery. Langmuir 2022, 38, 13330–13343. [Google Scholar] [CrossRef] [PubMed]
- Petkov, J.T.; Penfold, J.; Thomas, R.K. Surfactant self-assembly structures and multilayer formation at the solid-solution interface induces by electrolyte, polymers and proteins. Curr. Opin. Colloid Interface Sci. 2021, 57, 101541. [Google Scholar] [CrossRef]
- Visaveliya, N.R.; Köhler, J.M. Single-Step in Situ Assembling Routes for the Shape Control of Polymer Nanoparticles. Biomacromolecules 2018, 19, 1047–1064. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Zhang, Y.; Weisensee, K. Conducting polymeric nanocomposites with a three-dimensional co-flow microfluidics platform. Micromachines 2019, 10, 383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tresset, G.; Marculescu, C.; Salonen, A.; Ni, M.; Iliescu, C. Fine Control Over the Size of Surfactant–Polyelectrolyte Nanoparticles by Hydrodynamic Flow Focusing. Anal. Chem. 2013, 85, 5850–5856. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.-P.; Grigsby, C.L.; Zhao, F.; Leong, K.W. Tuning Physical Properties of Nanocomplexes through Microfluidics-Assisted Confinement. Nano Lett. 2011, 11, 2178–2182. [Google Scholar] [CrossRef] [Green Version]
- Sevim, S.; Sorrenti, A.; Franco, C.; Furukawa, S.; Pané, S.; Demello, A.J.; Puigmartí-Luis, J. Self-assembled materials and supramolecular chemistry within microfluidic environments: From common thermodynamic states to non-equilibrium structures. Chem. Soc. Rev. 2018, 47, 3788–3803. [Google Scholar] [CrossRef] [Green Version]
- Arnon, Z.A.; Vitalis, A.; Levin, A.; Michaels, T.C.T.; Caflisch, A.; Knowles, T.P.J.; Adler-Abramovich, L.; Gazit, E. Dynamic microfluidic control of supramolecular peptide self-assembly. Nat. Commun. 2016, 7, 13190. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Chen, Q.; Ma, Y.; Sun, J. Microfluidic Methods for Fabrication and Engineering of Nanoparticle Drug Delivery Systems. ACS Appl. Bio Mater. 2020, 3, 107–120. [Google Scholar] [CrossRef]
- Maeki, M.; Kimura, N.; Sato, Y.; Harashima, H.; Tokeshi, M. Advances in microfluidics for lipid nanoparticles and extracellular vesicles and applications in drug delivery systems. Adv. Drug Deliv. Rev. 2018, 128, 84–100. [Google Scholar] [CrossRef]
- Chiesa, E.; Dorati, R.; Pisani, S.; Conti, B.; Bergamini, G.; Modena, T.; Genta, I. The Microfluidic Technique and the Manufacturing of Polysaccharide Nanoparticles. Pharmaceutics 2018, 10, 267. [Google Scholar] [CrossRef] [PubMed]
- Seiffert, S. Microfluidics and Macromolecules: Top-Down Analytics and Bottom-Up Engineering of Soft Matter at Small Scales. Macromol. Chem. Phys. 2017, 218, 1600280. [Google Scholar] [CrossRef]
- Sun, C.; Zhang, M.; Huang, G.; Zhang, P.; Lin, R.; Wang, X.; You, H. A Microfluidic System of Gene Transfer by Ultrasound. Micromachines 2022, 13, 1126. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Lee, S.M.-Y.; Yi, C.; Li, C.-W. Controllable synthesis of functional nanoparticles by microfluidic platforms for biomedical applications—A review. Lab A Chip 2017, 17, 209–226. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Silva, T.C.; van Opstal, A.; Romeijn, S.; Every, H.A.; Jiskoot, W.; Witkamp, G.-J.; Ottens, M. The Investigation of Protein Diffusion via H-Cell Microfluidics. Biophys. J. 2019, 116, 595–609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sonker, M.; Sahore, V.; Woolley, A.T. Recent advances in microfluidic sample preparation and separation techniques for molecular biomarker analysis: A critical review. Anal. Chim. Acta 2017, 986, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Martins, C.; Araújo, F.; Gomes, M.J.; Fernandes, C.; Nunes, R.; Li, W.; Santos, H.A.; Borges, F.; Sarmento, B. Using microfluidic platforms to develop CNS-targeted polymeric nanoparticles for HIV therapy. Eur. J. Pharm. Biopharm. 2019, 138, 111–124. [Google Scholar] [CrossRef]
- Anna, S.L. Droplets and bubbles in microfluidic devices. In Annual Review of Fluid Mechanics; Annual Reviews: San Mateo, CL, USA, 2016; Volume 48, pp. 285–309. [Google Scholar]
- Dreyfus, R.; Tabeling, P.; Willaime, H. Ordered and Disordered Patterns in Two-Phase Flows in Microchannels. Phys. Rev. Lett. 2003, 90, 144505. [Google Scholar] [CrossRef] [Green Version]
- Anna, S.L.; Bontoux, N.; Stone, H.A. Formation of dispersions using “flow focusing” in microchannels. Appl. Phys. Lett. 2003, 82, 364–366. [Google Scholar] [CrossRef]
- Cohen, C.; Giles, R.; Sergeyeva, V.; Mittal, N.; Tabeling, P.; Zerrouki, D.; Baudry, J.; Bibette, J.; Bremond, N. Parallelised production of fine and calibrated emulsions by coupling flow-focusing technique and partial wetting phenomenon. Microfluid. Nanofluidics 2014, 17, 959–966. [Google Scholar] [CrossRef]
- Christopher, G.F.; Anna, S.L. Microfluidic methods for generating continuous droplet streams. J. Phys. D Appl. Phys. 2007, 40, R319–R336. [Google Scholar] [CrossRef]
- Song, H.; Chen, D.L.; Ismagilov, R.F. Reactions in Droplets in Microfluidic Channels. Angew. Chem. Int. Ed. Engl. 2006, 45, 7336–7356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, H.; Tice, J.D.; Ismagilov, R.F. A Microfluidic System for Controlling Reaction Networks in Time. Angew. Chem. Int. Ed. 2003, 42, 768–772. [Google Scholar] [CrossRef] [PubMed]
- Shao, C.; Chi, J.; Shang, L.; Fan, Q.; Ye, F. Droplet microfluidics-based biomedical microcarriers. Acta Biomater. 2022, 138, 21–33. [Google Scholar] [CrossRef]
- Gwon, S.; Park, S. Preparation of uniformly sized interpenetrating polymer network polyelectrolyte hydrogel droplets from a solid-state liquid crystal shell. J. Ind. Eng. Chem. 2021, 99, 235–245. [Google Scholar] [CrossRef]
- Wanselius, M.; Rodler, A.; Searle, S.S.; Abrahmsén-Alami, S.; Hansson, P. Responsive Hyaluronic Acid–Ethylacrylamide Microgels Fabricated Using Microfluidics Technique. Gels 2022, 8, 588. [Google Scholar] [CrossRef]
- Link, D.R.; Anna, S.I.; Weitz, D.A.; Stone, H.A. Geometrically Mediated Breakup of Drops in Microfluidic Devices. Phys. Rev. Lett. 2004, 92, 545031–545034. [Google Scholar] [CrossRef] [Green Version]
- Anna, S.L.; Mayer, H.C. Microscale tipstreaming in a microfluidic flow focusing device. Phys. Fluids 2006, 18, 121512. [Google Scholar] [CrossRef] [Green Version]
- Lee, W.; Walker, L.M.; Anna, S.L. Role of geometry and fluid properties in droplet and thread formation processes in planar flow focusing. Phys. Fluids 2009, 21, 032103. [Google Scholar] [CrossRef]
- Effect of soluble surfactant on the motion of a confined droplet in a square microchannel. Phys. Fluids 2019, 31, 117104. [CrossRef]
- Dogishi, Y.; Endo, S.; Sohn, W.Y.; Katayama, K. Formation of Photo-Responsive Liquid Crystalline Emulsion by Using Microfluidics Device. Entropy 2017, 19, 669. [Google Scholar] [CrossRef]
- Hennequin, Y.; Pannacci, N.; de Torres, C.P.; Tetradis-Meris, G.; Chapuliot, S.; Bouchaud, E.; Tabeling, P. Synthesizing Microcapsules with Controlled Geometrical and Mechanical Properties with Microfluidic Double Emulsion Technology. Langmuir 2009, 25, 7857–7861. [Google Scholar] [CrossRef] [PubMed]
- Patel, L.; Mansour, O.T.; Bryant, H.; Abdullahi, W.; Dalgliesh, R.M.; Griffiths, P.C. Interaction of Low Molecular Weight Poly(diallyldimethylammonium chloride) and Sodium Dodecyl Sulfate in Low Surfactant–Polyelectrolyte Ratio, Salt-Free Solutions. Langmuir 2020, 36, 8815–8825. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Prajapati, R.; Bhattacharya, A.; Mukherjee, T.K. Microscopic Evidence of “Necklace and Bead”-Like Morphology of Polymer–Surfactant Complexes: A Comparative Study on Poly(vinylpyrrolidone)–Sodium Dodecyl Sulfate and Poly(diallyldimethylammonium chloride)–Sodium Dodecyl Sulfate Systems. Langmuir 2014, 30, 9859–9865. [Google Scholar] [CrossRef] [PubMed]
- Poghosyan, A.H.; Arsenyan, L.H.; Gharabekyan, H.H.; Koetz, J.; Shahinyan, A.A. Molecular dynamics study of poly(diallyldimethylammonium chloride) (PDADMAC)/sodium dodecyl sulfate (SDS)/decanol/water systems. J. Phys. Chem. B 2009, 113, 1303–1310. [Google Scholar] [CrossRef]
- Lindman, B.; Puyal, M.C.; Kamenka, N.; Rymdén, R.; Stilbs, P. Micelle formation of anionic and cationic surfactants from Fourier transform hydrogen-1 and lithium-7 nuclear magnetic resonance and tracer self-diffusion studies. J. Phys. Chem. A 1984, 88, 5048–5057. [Google Scholar]
- Gurkov, T.D.; Dimitrova, D.T.; Marinova, K.G.; Bilke-Crause, C.; Gerber, C.; Ivanov, I.B. Ionic surfactants on fluid interfaces: Determination of the adsorption; role of the salt and the type of the hydrophobic phase. Colloids Surfaces A Physicochem. Eng. Asp. 2005, 261, 29–38. [Google Scholar] [CrossRef]
- Carlsson, S.; Liljeroth, P.; Kontturi, K. Channel flow configuration for studying the kinetics of surfactant-poly electrolyte binding. Anal. Chem. 2005, 77, 6895–6901. [Google Scholar] [CrossRef]
- Grueso, E.; Roldan, E.; Sanchez, F. Kinetic Study of the Cetyltrimethylammonium/DNA Interaction. J. Phys. Chem. B 2009, 113, 8319–8323. [Google Scholar] [CrossRef]
- McDonald, J.C.; Duffy, D.C.; Anderson, J.R.; Chiu, D.T.; Wu, H.; Schueller, O.J.A.; Whitesides, G.M. Fabrication of microfluidic systems in poly(dimethylsiloxane). Electrophoresis 2000, 21, 27–40. [Google Scholar] [CrossRef]
- Shilova, S.V.; Bezrukov, A.N.; Tret’Yakova, A.Y.; Voronin, M.A.; Zakharova, L.Y.; Barabanov, V.P. Effect of the length of surfactant hydrocarbon radicals on the association of cationic polyelectrolytes with alkyl sulfates in water-alcohol solutions. Polym. Sci. Ser. A 2012, 54, 19–25. [Google Scholar] [CrossRef]
- Bezrukov, A.; Galyametdinov, Y. Characterizing properties of polymers and colloids by their reaction-diffusion behavior in microfluidic channels. Colloids Surf. A Physicochem. Eng. Asp. 2021, 630, 1273–1288. [Google Scholar] [CrossRef]
- Berthier, J.; Silberzan, P. Microfluidics for Biotechnology, 2nd ed.; Artech House: London, UK, 2009; p. 512. [Google Scholar]
- Panariello, L.; Mazzei, L.; Gavriilidis, A. Modelling the synthesis of nanoparticles in continuous microreactors: The role of diffusion and residence time distribution on nanoparticle characteristics. Chem. Eng. J. 2018, 350, 1144–1154. [Google Scholar] [CrossRef] [Green Version]
- Bezrukov, A.; Galyametdinov, Y. On-Chip Control over Polyelectrolyte–Surfactant Complexation in Nonequilibrium Microfluidic Confinement. Polymers 2022, 14, 4109. [Google Scholar] [CrossRef] [PubMed]
- Iliescu, C.; Tresset, G. Microfluidics-Driven Strategy for Size-Controlled DNA Compaction by Slow Diffusion through Water Stream. Chem. Mater. 2015, 27, 8193–8197. [Google Scholar] [CrossRef]
- Iliescu, C.; Mărculescu, C.; Venkataraman, S.; Languille, B.; Yu, H.; Tresset, G. On-Chip Controlled Surfactant–DNA Coil–Globule Transition by Rapid Solvent Exchange Using Hydrodynamic Flow Focusing. Langmuir 2014, 30, 13125–13136. [Google Scholar] [CrossRef] [PubMed]
- Henrik, B. Theoretical Microfluidics; Oxford University Press: Oxford, UK, 2007; p. 288. [Google Scholar]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bezrukov, A.; Galyametdinov, Y. Tuning Properties of Polyelectrolyte-Surfactant Associates in Two-Phase Microfluidic Flows. Polymers 2022, 14, 5480. https://doi.org/10.3390/polym14245480
Bezrukov A, Galyametdinov Y. Tuning Properties of Polyelectrolyte-Surfactant Associates in Two-Phase Microfluidic Flows. Polymers. 2022; 14(24):5480. https://doi.org/10.3390/polym14245480
Chicago/Turabian StyleBezrukov, Artem, and Yury Galyametdinov. 2022. "Tuning Properties of Polyelectrolyte-Surfactant Associates in Two-Phase Microfluidic Flows" Polymers 14, no. 24: 5480. https://doi.org/10.3390/polym14245480