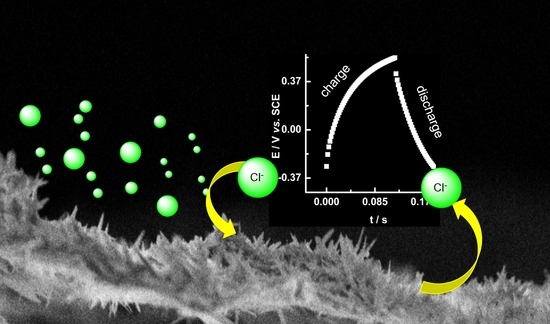
Capacitors Based on Polypyrrole Nanowire Electrodeposits
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martinez-Rojas, F.; Castaneda, E.; Armijo, F. Conducting polymer applied in a label-free electrochemical immunosensor for the detection prostate-specific antigen using its redox response as an analytical signal. J. Electroanal. Chem. 2021, 880, 114877. [Google Scholar] [CrossRef]
- Martinez-Rojas, F.; Diculescu, V.C.; Armijo, F. Electrochemical Immunosensing Platform for the Determination of the 20S Proteasome Using an Aminophenylboronic/Poly-indole-6-carboxylic Acid-Modified Electrode. ACS Appl. Bio. Mater. 2020, 3, 4941–4948. [Google Scholar] [CrossRef] [PubMed]
- Friend, R.H.; Gymer, R.W.; Holmes, A.B.; Burroughes, J.H.; Marks, R.N.; Taliani, C.; Bradley, D.D.C.; Dos Santos, D.A.; Bredas, J.L.; Logdlund, M.; et al. Electroluminescence in conjugated polymers. Nature 1999, 397, 121–128. [Google Scholar] [CrossRef]
- Ramirez, A.M.; Cattin, L.; Bernede, J.C.; Diaz, F.R.; Gacitua, M.A.; del Valle, M.A. Nanostructured TiO2 and PEDOT Electrodes with Photovoltaic Application. Nanomaterials 2021, 11, 107. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.-M.; Jiang, K.-J.; Zhang, Y.; Yu, G.-H.; Gao, C.-Y.; Fan, X.-H.; Yang, L.-M. In-Situ polymerization of PEDOT in perovskite Thin films for efficient and stable photovoltaics. Chem. Eng. J. 2022, 430, 133109. [Google Scholar] [CrossRef]
- Kim, J.; Lee, S.H.; Park, C.; Kim, H.S.; Park, J.H.; Chung, K.Y.; Ahn, H. Controlling Vanadate Nanofiber Interlayer via Intercalation with Conducting Polymers: Cathode Material Design for Rechargeable Aqueous Zinc Ion Batteries. Adv. Funct. Mater. 2021, 31, 2100005. [Google Scholar] [CrossRef]
- Jung, Y.J.; Singh, N.; Choi, K.S. Cathodic Deposition of Polypyrrole Enabling the One-Step Assembly of Metal-Polymer Hybrid Electrodes. Angew. Chem. Int. Ed. 2009, 48, 8331–8334. [Google Scholar] [CrossRef]
- Huang, X.H.; Tu, J.P.; Xia, X.H.; Wang, X.L.; Xiang, J.Y.; Zhang, L. Porous NiO/poly(3,4-ethylenedioxythiophene) films as anode materials for lithium ion batteries. J. Power Sources 2010, 195, 1207–1210. [Google Scholar] [CrossRef]
- Ma, X.M.; Zhou, W.Q.; Mo, D.Z.; Hou, J.; Xu, J.K. Effect of substituent position on electrodeposition, morphology, and capacitance performance of polyindole bearing a carboxylic group. Electrochim. Acta 2015, 176, 1302–1312. [Google Scholar] [CrossRef]
- Liu, W.N.; Li, X.X.; Li, W.J.; Ye, Y.M.; Wang, H.; Su, P.P.; Yang, W.Y.; Yang, Y. High-performance supercapacitors based on free-standing SiC@PEDOT nanowires with robust cycling stability. J. Energy Chem. 2022, 66, 30–37. [Google Scholar] [CrossRef]
- Jeon, J.W.; Ma, Y.G.; Mike, J.F.; Shao, L.; Balbuena, P.B.; Lutkenhaus, J.L. Oxidatively stable polyaniline:polyacid electrodes for electrochemical energy storage. Phys. Chem. Chem. Phys. 2013, 15, 9654–9662. [Google Scholar] [CrossRef]
- Lota, K.; Lota, G.; Sierczynska, A.; Acznik, I. Carbon/polypyrrole composites for electrochemical capacitors. Synth. Met. 2015, 203, 44–48. [Google Scholar] [CrossRef]
- Eftekhari, A.; Li, L.; Yang, Y. Polyaniline supercapacitors. J. Power Sources 2017, 347, 86–107. [Google Scholar] [CrossRef]
- Tietje-Girault, J.; Ponce de Leon, C.; Walsh, F.C. Electrochemically deposited polypyrrole films and their characterization. Surf. Coat. Tech. 2007, 201, 6025–6034. [Google Scholar] [CrossRef]
- Canoluk, C.; Gursoy, S.S. Chemical modification of rose leaf with polypyrrole for the removal of Pb (II) and Cd (II) from aqueous solution. J. Macromol. Sci. A 2017, 54, 782–790. [Google Scholar] [CrossRef]
- Zhou, M.; Heinze, J. Electropolymerization of pyrrole and electrochemical study of polypyrrole. 2. Influence of acidity on the formation of polypyrrole and the multipathway mechanism. J. Phys. Chem. B 1999, 103, 8443–8450. [Google Scholar] [CrossRef]
- Funt, B.L.; Diaz, A.F. Organic Electrochemistry: An Introduction and a Guide; Marcel Dekker: New York, NY, USA, 1991; p. 1337. [Google Scholar]
- Genies, E.M.; Bidan, G.; Diaz, A.F. Spectroelectrochemical study of polypyrrole films. J. Electroanal. Chem. 1983, 149, 101. [Google Scholar] [CrossRef]
- del Valle, M.A.; Ramirez, A.M.R.; Diaz, F.R.; Pardo, M.A.; Ortega, E.; Armijo, F. Influence of the Electrolyte Salt on the Electrochemical Polymerization of Pyrrole. Effects on p-Doping/Undoping, Conductivity and Morphology. Int. J. Electrochem. Sci. 2018, 13, 12404–12419. [Google Scholar] [CrossRef]
- Bae, J.; Park, J.Y.; Kwon, O.S.; Lee, C.S. Energy efficient capacitors based on graphene/conducting polymer hybrids. J. Ind. Eng. Chem. 2017, 51, 1–11. [Google Scholar] [CrossRef]
- Gan, J.K.; Lim, Y.S.; Pandikumar, A.; Huang, N.M.; Lim, H.N. Graphene/polypyrrole-coated carbon nanofiber core-shell architecture electrode for electrochemical capacitors. RSC Adv. 2015, 5, 12692–12699. [Google Scholar] [CrossRef]
- Ramírez, A.M.R.; Gacitúa, M.A.; Ortega, E.; Díaz, F.R.; del Valle, M.A. Electrochemical in situ synthesis of polypyrrole nanowires. Electrochem. Commun. 2019, 102, 94–98. [Google Scholar] [CrossRef]
- Walcarius, A.; Sibottier, E.; Etienne, M.; Ghanbaja, J. Electrochemically assisted self-assembly of mesoporous silica thin films. Nat. Mater. 2007, 6, 602–608. [Google Scholar] [CrossRef] [PubMed]
- Walcarius, A. Electroinduced Surfactant Self-Assembly Driven to Vertical Growth of Oriented Mesoporous Films. Acc. Chem. Res. 2021, 54, 3563–3575. [Google Scholar] [CrossRef] [PubMed]
- Ullah, W.; Herzog, G.; Vila, N.; Walcarius, A. Electrografting and electropolymerization of nanoarrays of PAni filaments through silica mesochannels. Electrochem. Commun. 2021, 122, 106896. [Google Scholar] [CrossRef]
- Hernandez, L.A.; del Valle, M.A.; Diaz, F.R.; Fermin, D.J.; Risbridger, T.A.G. Polymeric nanowires directly electrosynthesized on the working electrode. Electrochim. Acta 2015, 166, 163–167. [Google Scholar] [CrossRef]
- Hernandez, L.A.; del Valle, M.A.; Armijo, F. Electrosynthesis and characterization of nanostructured polyquinone for use in detection and quantification of naturally occurring dsDNA. Biosens. Bioelectron. 2016, 79, 280–287. [Google Scholar] [CrossRef]
- Ramirez, M.R.A.; del Valle, M.A.; Armijo, F.; Diaz, F.R.; Pardo, M.A.; Ortega, E. Enhancement of electrodes modified by electrodeposited PEDOT-nanowires with dispersed Pt nanoparticles for formic acid electro-oxidation. J. Appl. Polym. Sci. 2017, 134, 44723–44729. [Google Scholar] [CrossRef]
- East, G.A.; del Valle, M.A. Easy-to-make Ag/AgCl reference electrode. J. Chem. Educ. 2000, 77, 97. [Google Scholar] [CrossRef]
- Mo, D.Z.; Zhou, W.Q.; Ma, X.M.; Xu, J.K.; Zhu, D.H.; Lu, B.Y. Electrochemical synthesis and capacitance properties of a novel poly(3,4-ethylenedioxythiophene bis-substituted bithiophene) electrode material. Electrochim. Acta 2014, 132, 67–74. [Google Scholar] [CrossRef]
- Del Valle, M.A.; Ramirez, A.M.; Hernandez, L.A.; Armijo, F.; Diaz, F.R.; Arteaga, G.C. Influence of the Supporting Electrolyte on the Electrochemical Polymerization of 3,4-Ethylenedioxythiophene. Effect on p- and n-Doping/Undoping, Conductivity and Morphology. Int. J. Electrochem. Sci. 2016, 11, 7048–7065. [Google Scholar] [CrossRef]
- Zhou, W.Q.; Ma, X.M.; Jiang, F.X.; Zhu, D.H.; Xu, J.K.; Lu, B.Y.; Liu, C.C. Electrochemical fabrication of a porous network MnO2/poly(5-cyanoindole) composite and its capacitance performance. Electrochim. Acta 2014, 138, 270–277. [Google Scholar] [CrossRef]
- Yang, X.; Liu, A.; Zhao, Y.; Lu, H.; Zhang, Y.; Wei, W.; Li, Y.; Liu, S. Three-Dimensional Macroporous Polypyrrole-Derived Graphene Electrode Prepared by the Hydrogen Bubble Dynamic Template for Supercapacitors and Metal-Free Catalysts. ACS Appl. Mater. Interfaces 2015, 7, 23731–23740. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, X.S. Conducting Polymers Directly Coated on Reduced Graphene Oxide Sheets as High-Performance Supercapacitor Electrodes. J. Phys. Chem. C 2012, 116, 5420–5426. [Google Scholar] [CrossRef]
- Weerasinghe, W.A.D.S.S.; Vidanapathirana, K.P.; Perera, K.S.; Bandaranayake, C.M. Effect of polymerization current density of electrodes on the performance of polypyrrole based redox-capacitor. J. Natl. Sci. Found. Sri Lanka 2017, 45, 73–77. [Google Scholar] [CrossRef] [Green Version]
- Del Valle, M.A.; Ramirez, A.; Hernandez, L.; Gacitua, M.A. Batteries Rechargeable by Solar Power, Based on Nanostructured Polymers (Chile Patent No. I. N. d. P. I. I. (Chile). 2018. Available online: https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2020107131 (accessed on 10 November 2022).
- Del Valle, M.A.; Gacitua, M.A.; Hernandez, L.A.; Díaz, F.R. Electrosynthesis of Polymer Nanothreads Directly onto Solid Surfaces (Electrodes) (Chile Patent No. I. N. d. P. I. I. (Chile). 2018. Available online: https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2020107130 (accessed on 10 November 2022).
- Del Valle, M.A.; Ramos, A.C.; Diaz, F.R.; Gacitua, M.A. Electrosynthesis and Characterisation of Polymer Nanowires from Thiophene and its Oligomers. J. Braz. Chem. Soc. 2015, 26, 2313–2320. [Google Scholar]
- Del Valle, M.A.; Hernández, L.A.; Díaz, F.R.; Ramos, A.C. Electrosynthesis and Characterization of Poly(3,4- ethylenedioxythiophene) Nanowires. Int. J. Electrochem. Sci. 2015, 10, 5152–5163. [Google Scholar]
- Del Valle, M.A.; Gacitua, M.; Diaz, F.R.; Armijo, F.; Soto, J.P. Electro-synthesis and characterization of polythiophene nanowires/platinum nano-particles composite electrodes. Study of formic acid electro-catalytic oxidation. Electrochim. Acta 2012, 71, 277–282. [Google Scholar] [CrossRef]
- Del Valle, M.A.; Gacitua, M.A.; Diaz, F.R.; Armijo, J.F.; del Rio, R.R. Electrosynthesis of polythiophene nanowires via mesoporous silica thin film templates. Electrochem. Commun. 2009, 11, 2117–2120. [Google Scholar] [CrossRef]
- Del Valle, M.A.; Salgado, R.; Armijo, F. PEDOT Nanowires and Platinum Nanoparticles Modified Electrodes to be Assayed in Formic Acid Electro-oxidation. Int. J. Electrochem. Sci. 2014, 9, 1557–1564. Available online: http://www.electrochemsci.org/papers/vol9/90301557.pdf (accessed on 10 November 2022).
- Zhou, M.; Heinze, J. Electropolymerization of pyrrole and electrochemical study of polypyrrole: 1. Evidence for structural diversity of polypyrrole. Electrochim. Acta 1999, 44, 1733–1748. [Google Scholar] [CrossRef]
- Wu, T.M.; Lin, S.H. Characterization and electrical properties of polypyrrole/multiwalled carbon nanotube composites synthesized by in situ chemical oxidative polymerization. J. Polym. Sci. Pol. Phys. 2006, 44, 1413–1418. [Google Scholar] [CrossRef]
- Mansour, A.E.; Valencia, A.M.; Lungwitz, D.; Wegner, B.; Tanaka, N.; Shoji, Y.; Fukushima, T.; Opitz, A.; Cocchi, C.; Koch, N. Understanding the evolution of the Raman spectra of molecularly p-doped poly(3-hexylthiophene-2,5-diyl): Signatures of polarons and bipolarons. Phys. Chem. Chem. Phys. 2022, 24, 3109–3118. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Burke, A.F. Review on supercapacitors: Technologies and performance evaluation. J. Energy Chem. 2021, 59, 276–291. [Google Scholar] [CrossRef]
- Hui, N.; Wang, J.; Liang, A.; Jiang, M. Conducting polyaniline nanowire arrays modified electrode for high performance supercapacitor and enhanced catalysis of nitrite reduction. Electroanalysis 2016, 28, 2979–2984. [Google Scholar] [CrossRef]
- Tran, C.B.; Zondaka, Z.; Le, Q.B.; Velmurugan, B.K.; Kiefer, R. Polypyrrole with Phosphor Tungsten Acid and Carbide-Derived Carbon: Change of Solvent in Electropolymerization and Linear Actuation. Materials 2021, 14, 6302. [Google Scholar] [CrossRef]
- Torop, J.; Aabloo, A.; Jagera, W.H.E. Novel actuators based on polypyrrole/carbide-derived carbon hybrid materials. Carbon 2014, 80, 387. [Google Scholar] [CrossRef] [Green Version]
- Khuyen, N.Q.; Kiefer, R.; Zondaka, Z.; Anbarjafari, G.; Peikolainen, A.-L.; Otero, T.F.; Tamm, T. Multifunctionality of Polypyrrole Polyethyleneoxide Composites: Concurrent Sensing, Actuation and Energy Storage. Polymers 2020, 12, 2060. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, Z.; Huang, Y.; Ma, Y.; Wang, C.; Chen, M.; Chen, Y. Supercapacitor Devices Based on Graphene Materials. J. Phys. Chem. C 2009, 113, 13103–13107. [Google Scholar] [CrossRef]





| Electrode | Ep 1/V | Qd 2 (mC cm−2) | Qud 3 (mC cm−2) | Qd/Qud 4 |
|---|---|---|---|---|
| PPy bulk | 0.60 | 0.10 | 0.10 | 1.04 |
| PPy-nw | 0.42 | 31.8 | 30.5 | 1.04 |
| Potentiodynamic | ||||||||
| Scan Rates (mV s−1) | 10 | 20 | 50 | 75 | 100 | 150 | ||
| PPy bulk | Specific Capacitance (F g−1) | 1.05 | 0.72 | 0.55 | 0.44 | 0.36 | 0.29 | |
| PPy-nw | 102.6 | 75.7 | 59.7 | 50.9 | 43.3 | 37.2 | ||
| Galvanostatic | ||||||||
| Current (mA) | 0.05 | 0.10 | 0.15 | |||||
| PPy bulk | Specific Capacitance (F g−1) | 4.86 | 3.84 | 3.49 | ||||
| PPy-nw | 263.9 | 217.4 | 200.9 | |||||
| Electrode Architecture | Perturbation Type and Intensity | Electrolyte | Specific Capacitance | Capacitance Retention%, Charge Cycle Number and Charge Potential | Ref. |
|---|---|---|---|---|---|
| GC|GO/PPy 1 | G 3: 1 mA cm−2 | KCl | 196 mF cm−1 | 90% for 500 cycles and 1.0 V | [33] |
| Au|GO/PPy 2 | G 3: 0.3 A g−1 | H2SO4 | 249 F g−1 | 81% for 1000 cycles and 1.0 V | [34] |
| FTO|PPy 1 | P 4: 20 mV s−1 | SDBS | 12 F g−1 | - | [35] |
| FTO|PPy 1 | G 3 2 mA cm−2 | SDBS | 7 F g−1 | - | [35] |
| PPyCDC-EG 1 | G 3 2 A g−1 | NaClO4 | 190 F g−1 | 90% for 1000 cycles and 0.3 V | [48] |
| PPy(SDBS)CDC 1 | P 4: 10 mV s−1 | SDBS | 131 F g−1 | - | [49] |
| PPy-PEO/DBS 1 | G 3 0.24 Ag−1 | NaCl | 100 F g−1 | - | [50] |
| ITO|nw-PPy 1 | G 3: 0.15 mA | LiCl | 291 F g−1 | 76.5% for 1000 cycles and 0.77 V | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramírez, A.M.R.; del Valle, M.A.; Ortega, E.; Díaz, F.R.; Gacitúa, M.A. Capacitors Based on Polypyrrole Nanowire Electrodeposits. Polymers 2022, 14, 5476. https://doi.org/10.3390/polym14245476
Ramírez AMR, del Valle MA, Ortega E, Díaz FR, Gacitúa MA. Capacitors Based on Polypyrrole Nanowire Electrodeposits. Polymers. 2022; 14(24):5476. https://doi.org/10.3390/polym14245476
Chicago/Turabian StyleRamírez, A. M. R., M. A. del Valle, E. Ortega, F. R. Díaz, and M. A. Gacitúa. 2022. "Capacitors Based on Polypyrrole Nanowire Electrodeposits" Polymers 14, no. 24: 5476. https://doi.org/10.3390/polym14245476