Biopolymer-Based Wound Dressings with Biochemical Cues for Cell-Instructive Wound Repair
Abstract
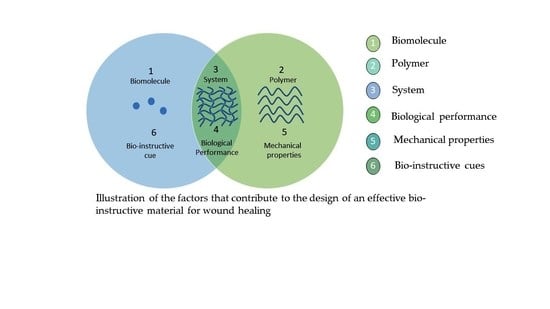
:1. Introduction
2. The Phases of Wound Healing

3. Functional Biopolymer-Based Wound Dressings
3.1. Chitin and Chitosan
3.2. Starch
3.3. Alginate
3.4. Cellulose
3.5. Carrageenan
3.6. Pectin
3.7. Hyaluronic Acid (HA)
3.8. Collagen
3.9. Gelatin
3.10. Silk
3.11. Keratin
4. Biochemical Cues in Wound Healing Applications
4.1. Peptides as Pioneering Materials for Wound Healing Applications
4.1.1. Antimicrobial Peptides
4.1.2. Collagen Mimetic Peptides
Collagen Mimetic Peptides with Integrin Targeting Motifs
4.2. Collagen Matrices Embedded with Biochemical Cues for the Promotion of Wound Healing
4.3. Cells as Directing Prompts for Enhanced Wound Healing
4.4. Decellularized Matrices as Regenerative Biomaterials for Wound Healing
4.5. Platelet-Rich Plasma in Progressive Wound Healing Applications
4.6. Delivery of Biometals for Wound Healing Applications
5. Further Perspectives
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Castaño, O.; Pérez-Amodio, S.; Navarro-Requena, C.; Mateos-Timoneda, M.; Engel, E. Instructive microenvironments in skin wound healing: Biomaterials as signal releasing platforms. Adv. Drug Deliv. Rev. 2018, 129, 95–117. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, N.K.; Kumar, S.S.D.; Houreld, N.N.; Abrahamse, H. A review on nanoparticle based treatment for wound healing. J. Drug Deliv. Sci. Technol. 2018, 44, 421–430. [Google Scholar] [CrossRef]
- Lindholm, C.; Searle, R. Wound management for the 21st century: Combining effectiveness and efficiency. Int. Wound J. 2016, 13, 5–15. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez, A.C.D.O.; Costa, T.F.; de Araújo Andrade, Z.; Medrado, A.R.A.P. Wound Healing—A Literature Review. An. Bras. Dermatol. 2016, 91, 614–620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collins, K.L.; Gates, E.M.; Gilchrist, C.L.; Hoffman, B.D. Bio-Instructive Cues in Scaffolds for Musculoskeletal Tissue Engineering and Regenerative Medicine. In Bio-Instructive Scaffolds for Musculoskeletal Tissue Engineering and Regenerative Medicine; Academic Press: London, UK, 2017; pp. 3–35. [Google Scholar]
- Sousa, C.; Saraiva, C.; Correia, T.; Pesqueira, T.; Patrício, S.; Rial-Hermida, M.; Borges, J.; Mano, J. Bioinstructive Layer-by-Layer-Coated Customizable 3D Printed Perfusable Microchannels Embedded in Photocrosslinkable Hydrogels for Vascular Tissue Engineering. Biomolecules 2021, 11, 863. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Fernandez, T.; Sikorski, P.; Leach, J.K. Bio-instructive materials for musculoskeletal regeneration. Acta Biomater. 2019, 96, 20–34. [Google Scholar] [CrossRef]
- Sharma, P.; Kumar, A.; Dey, A.D.; Behl, T.; Chadha, S. Stem cells and growth factors-based delivery approaches for chronic wound repair and regeneration: A promise to heal from within. Life Sci. 2021, 268, 118932. [Google Scholar] [CrossRef]
- Vowden, K.; Vowden, P. Wound dressings: Principles and practice. Surg. (Oxford) 2017, 35, 489–494. [Google Scholar] [CrossRef]
- Rivero, G.; Popov Pereira da Cunha, M.D.; Caracciolo, P.C.; Abraham, G.A. Nanofibrous Scaffolds for Skin Tissue Engineering and Wound Healing Applications. In Tissue Engineering Using Ceramics and Polymers; Woodhead Publishing: Sawston, UK, 2022; pp. 645–681. [Google Scholar]
- Moholkar, D.N.; Sadalage, P.S.; Peixoto, D.; Paiva-Santos, A.C.; Pawar, K.D. Recent advances in biopolymer-based formulations for wound healing applications. Eur. Polym. J. 2021, 160, 110784. [Google Scholar] [CrossRef]
- Xiang, J.; Shen, L.; Hong, Y. Status and future scope of hydrogels in wound healing: Synthesis, materials and evaluation. Eur. Polym. J. 2020, 130, 109609. [Google Scholar] [CrossRef]
- Singh, S.; Young, A.; McNaught, C.-E. The physiology of wound healing. Surgery 2017, 35, 473–477. [Google Scholar] [CrossRef]
- Kordestani, S.S. Wound Healing Process. In Atlas of Wound Healing; Elsevier: Amsterdam, The Netherlands, 2019; p. 1. [Google Scholar]
- Qing, C. The molecular biology in wound healing & non-healing wound. Chin. J. Traumatol. 2017, 20, 189–193. [Google Scholar] [CrossRef]
- Li, L.; Yang, Y.; Yang, Z.; Zheng, M.; Luo, G.; He, W.; Yin, R. Effects of ALA-PDT on the macrophages in wound healing and its related mechanisms in vivo and in vitro. Photodiagnosis Photodyn. Ther. 2022, 38, 102816. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Huang, J.; Fang, Y.; Huang, H.; Wu, J. 1D, 2D, and 3D scaffolds promoting angiogenesis for enhanced wound healing. Chem. Eng. J. 2022, 437, 134690. [Google Scholar] [CrossRef]
- Spiller, S.; Clauder, F.; Bellmann-Sickert, K.; Beck-Sickinger, A.G. Improvement of wound healing by the development of ECM-inspired biomaterial coatings and controlled protein release. Biol. Chem. 2021, 402, 1271–1288. [Google Scholar] [CrossRef] [PubMed]
- Volk, S.W.; Iqbal, S.A.; Bayat, A. Interactions of the Extracellular Matrix and Progenitor Cells in Cutaneous Wound Healing. Adv. Wound Care 2013, 2, 261–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rangaraj, A.G.; Harding, K.G. Role of Collagen in Wound Management. Wounds UK 2011, 7, 2–8. [Google Scholar]
- Petkovic, M.; Mouritzen, M.V.; Mojsoska, B.; Jenssen, H. Immunomodulatory Properties of Host Defence Peptides in Skin Wound Healing. Biomolecules 2021, 11, 952. [Google Scholar] [CrossRef]
- Gardikiotis, I.; Cojocaru, F.-D.; Mihai, C.-T.; Balan, V.; Dodi, G. Borrowing the Features of Biopolymers for Emerging Wound Healing Dressings: A Review. Int. J. Mol. Sci. 2022, 23, 8778. [Google Scholar] [CrossRef]
- Agrawal, P.; Soni, S.; Mittal, G.; Bhatnagar, A. Role of Polymeric Biomaterials as Wound Healing Agents. Int. J. Low. Extremity Wounds 2014, 13, 180–190. [Google Scholar] [CrossRef]
- Chowdhary, K.; Rathore, S.P.S. Biopolymers for Wound Healing. Res. Reinf. 2015, 3, 1–8. [Google Scholar]
- Sahana, T.G.; Rekha, P.D. Biopolymers: Applications in wound healing and skin tissue engineering. Mol. Biol. Rep. 2018, 45, 2857–2867. [Google Scholar] [CrossRef] [PubMed]
- Arif, M.M.; Khan, S.M.; Gull, N.; Tabish, T.A.; Zia, S.; Khan, R.U.; Awais, S.M.; Butt, M.A. Polymer-based biomaterials for chronic wound management: Promises and challenges. Int. J. Pharm. 2021, 598, 120270. [Google Scholar] [CrossRef]
- Kaur, G.; Narayanan, G.; Garg, D.; Sachdev, A.; Matai, I. Biomaterials-Based Regenerative Strategies for Skin Tissue Wound Healing. ACS Appl. Bio Mater. 2022, 5, 2069–2106. [Google Scholar] [CrossRef] [PubMed]
- Kumar Dutta, P.; Dutta, J.; Tripathi, V.S. Chitin and Chitosan: Chemistry, Properties and Applications. J. Sci. Ind. Res. (India) 2004, 63, 20–31. [Google Scholar]
- Shivakumar, P.; Gupta, M.S.; Jayakumar, R.; Gowda, D.V. Prospection of chitosan and its derivatives in wound healing: Proof of patent analysis (2010–2020). Int. J. Biol. Macromol. 2021, 184, 701–712. [Google Scholar] [CrossRef]
- Bano, I.; Arshad, M.; Yasin, T.; Ghauri, M.A.; Younus, M. Chitosan: A potential biopolymer for wound management. Int. J. Biol. Macromol. 2017, 102, 380–383. [Google Scholar] [CrossRef]
- Nie, J.; Pei, B.; Wang, Z.; Hu, Q. Construction of ordered structure in polysaccharide hydrogel: A review. Carbohydr. Polym. 2018, 205, 225–235. [Google Scholar] [CrossRef]
- Pandey, A.; Sauraj; Ali, A.; Negi, Y. Synthesis of polygonal chitosan microcapsules for the delivery of amygdalin loaded silver nanoparticles in breast cancer therapy. Mater. Today Proc. 2021, 43, 3744–3748. [Google Scholar] [CrossRef]
- Li, N.; Yang, X.; Liu, W.; Xi, G.; Wang, M.; Liang, B.; Ma, Z.; Feng, Y.; Chen, H.; Shi, C. Tannic Acid Cross-linked Polysaccharide-Based Multifunctional Hemostatic Microparticles for the Regulation of Rapid Wound Healing. Macromol. Biosci. 2018, 18, e1800209. [Google Scholar] [CrossRef]
- Leonhardt, E.E.; Kang, N.; Hamad, M.A.; Wooley, K.L.; Elsabahy, M. Absorbable hemostatic hydrogels comprising composites of sacrificial templates and honeycomb-like nanofibrous mats of chitosan. Nat. Commun. 2019, 10, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torres, F.G.; Commeaux, S.; Troncoso, O.P. Starch-based biomaterials for wound-dressing applications. Starch-Stärke 2013, 65, 543–551. [Google Scholar] [CrossRef]
- Palanisamy, C.P.; Cui, B.; Zhang, H.; Gunasekaran, V.P.; Ariyo, A.L.; Jayaraman, S.; Rajagopal, P.; Long, Q. A critical review on starch-based electrospun nanofibrous scaffolds for wound healing application. Int. J. Biol. Macromol. 2022, 222, 1852–1860. [Google Scholar] [CrossRef]
- Gopinath, V.; Kamath, S.M.; Priyadarshini, S.; Chik, Z.; Alarfaj, A.A.; Hirad, A.H. Multifunctional applications of natural polysaccharide starch and cellulose: An update on recent advances. Biomed. Pharmacother. 2022, 146, 112492. [Google Scholar] [CrossRef]
- Mao, Q.; Hoffmann, O.; Yu, K.; Lu, F.; Lan, G.; Dai, F.; Shang, S.; Xie, R. Self-contracting oxidized starch/gelatin hydrogel for noninvasive wound closure and wound healing. Mater. Des. 2020, 194, 108916. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, X. Alginate hydrogel dressings for advanced wound management. Int. J. Biol. Macromol. 2020, 162, 1414–1428. [Google Scholar] [CrossRef]
- Chaudhary, R.; Nawaz, K.; Khan, A.K.; Hano, C.; Abbasi, B.H.; Anjum, S. An Overview of the Algae-Mediated Biosynthesis of Nanoparticles and Their Biomedical Applications. Biomolecules 2020, 10, 1498. [Google Scholar] [CrossRef] [PubMed]
- Varaprasad, K.; Jayaramudu, T.; Kanikireddy, V.; Toro, C.; Sadiku, E.R. Alginate-based composite materials for wound dressing application:A mini review. Carbohydr. Polym. 2020, 236, 116025. [Google Scholar] [CrossRef]
- Aderibigbe, B.A.; Buyana, B. Alginate in Wound Dressings. Pharmaceutics 2018, 10, 42. [Google Scholar] [CrossRef] [Green Version]
- Lu, W.; Bao, D.; Ta, F.; Liu, D.; Zhang, D.; Zhang, Z.; Fan, Z. Multifunctional Alginate Hydrogel Protects and Heals Skin Defects in Complex Clinical Situations. ACS Omega 2020, 5, 17152–17159. [Google Scholar] [CrossRef]
- Kanikireddy, V.; Varaprasad, K.; Jayaramudu, T.; Karthikeyan, C.; Sadiku, R. Carboxymethyl cellulose-based materials for infection control and wound healing: A review. Int. J. Biol. Macromol. 2020, 164, 963–975. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sui, Y.; Liu, C.; Liu, C.; Wu, M.; Li, B.; Li, Y. A physically crosslinked polydopamine/nanocellulose hydrogel as potential versatile vehicles for drug delivery and wound healing. Carbohydr. Polym. 2018, 188, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Yegappan, R.; Selvaprithiviraj, V.; Amirthalingam, S.; Jayakumar, R. Carrageenan based hydrogels for drug delivery, tissue engineering and wound healing. Carbohydr. Polym. 2018, 198, 385–400. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, M.; Noruzi, E.B.; Sheykhsaran, E.; Ebadi, B.; Kariminezhad, Z.; Molaparast, M.; Mehrabani, M.G.; Mehramouz, B.; Yousefi, M.; Ahmadi, R.; et al. Carbohydrate polymer-based silver nanocomposites: Recent progress in the antimicrobial wound dressings. Carbohydr. Polym. 2019, 231, 115696. [Google Scholar] [CrossRef]
- Noor, H.M. Global Health Management Journal Potential of Carrageenans in Foods and Medical Applications. J. Glob. Health 2018, 2, 32–38. [Google Scholar]
- Tavakoli, S.; Mokhtari, H.; Kharaziha, M.; Kermanpur, A.; Talebi, A.; Moshtaghian, J. A multifunctional nanocomposite spray dressing of Kappa-carrageenan-polydopamine modified ZnO/L-glutamic acid for diabetic wounds. Mater. Sci. Eng. C 2020, 111, 110837. [Google Scholar] [CrossRef]
- Munarin, F.; Tanzi, M.C.; Petrini, P. Advances in biomedical applications of pectin gels. Int. J. Biol. Macromol. 2012, 51, 681–689. [Google Scholar] [CrossRef]
- Eivazzadeh-Keihan, R.; Noruzi, E.B.; Aliabadi, H.A.M.; Sheikhaleslami, S.; Akbarzadeh, A.R.; Hashemi, S.M.; Gorab, M.G.; Maleki, A.; Cohan, R.A.; Mahdavi, M.; et al. Recent advances on biomedical applications of pectin-containing biomaterials. Int. J. Biol. Macromol. 2022, 217, 1–18. [Google Scholar] [CrossRef]
- Giusto, G.; Vercelli, C.; Comino, F.; Caramello, V.; Tursi, M.; Gandini, M. A new, easy-to-make pectin-honey hydrogel enhances wound healing in rats. BMC Complement. Altern. Med. 2017, 17, 266. [Google Scholar] [CrossRef] [Green Version]
- Graça, M.F.P.; Miguel, S.P.; Cabral, C.S.D.; Correia, I.J. Hyaluronic acid—Based wound dressings: A review. Carbohydr. Polym. 2020, 241, 116364. [Google Scholar] [CrossRef]
- Yang, H.; Song, L.; Zou, Y.; Sun, D.; Wang, L.; Yu, Z.; Guo, J. Role of Hyaluronic Acids and Potential as Regenerative Biomaterials in Wound Healing. ACS Appl. Bio. Mater. 2020, 4, 311–324. [Google Scholar] [CrossRef] [PubMed]
- Hussain, Z.; Thu, H.E.; Katas, H.; Bukhari, S.N.A. Hyaluronic Acid-Based Biomaterials: A Versatile and Smart Approach to Tissue Regeneration and Treating Traumatic, Surgical, and Chronic Wounds. Polym. Rev. 2017, 57, 594–630. [Google Scholar] [CrossRef]
- da Silva, L.P.; Santos, T.C.; Rodrigues, D.B.; Pirraco, R.P.; Cerqueira, M.T.; Reis, R.L.; Correlo, V.M.; Marques, A.P. Stem Cell-Containing Hyaluronic Acid-Based Spongy Hydrogels for Integrated Diabetic Wound Healing. J. Investig. Dermatol. 2017, 137, 1541–1551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, J.; Ren, J.; Chen, G.; Li, Z.; Liu, Y.; Wang, G.; Wu, X. Tunable sequential drug delivery system based on chitosan/hyaluronic acid hydrogels and PLGA microspheres for management of non-healing infected wounds. Mater. Sci. Eng. C 2018, 89, 213–222. [Google Scholar] [CrossRef]
- Strauss, K.; Chmielewski, J. Advances in the design and higher-order assembly of collagen mimetic peptides for regenerative medicine. Curr. Opin. Biotechnol. 2017, 46, 34–41. [Google Scholar] [CrossRef]
- Li, Y.; Yu, S.M. Targeting and mimicking collagens via triple helical peptide assembly. Curr. Opin. Chem. Biol. 2013, 17, 968–975. [Google Scholar] [CrossRef] [Green Version]
- Zheng, C.; Liu, X.; Luo, X.; Zheng, M.; Wang, X.; Dan, W.; Jiang, H. Development of a novel bio-inspired “cotton-like” collagen aggregate/chitin based biomaterial with a biomimetic 3D microstructure for efficient hemostasis and tissue repair. J. Mater. Chem. B 2019, 7, 7338–7350. [Google Scholar] [CrossRef]
- Duconseille, A.; Astruc, T.; Quintana, N.; Meersman, F.; Sante-Lhoutellier, V. Gelatin structure and composition linked to hard capsule dissolution: A review. Food Hydrocoll. 2015, 43, 360–376. [Google Scholar] [CrossRef]
- Mushtaq, F.; Raza, Z.A.; Batool, S.R.; Zahid, M.; Onder, O.C.; Rafique, A.; Nazeer, M.A. Preparation, properties, and applications of gelatin-based hydrogels (GHs) in the environmental, technological, and biomedical sectors. Int. J. Biol. Macromol. 2022, 218, 601–633. [Google Scholar] [CrossRef]
- Jeong, S.; Kim, B.; Park, M.; Ban, E.; Lee, S.-H.; Kim, A. Improved Diabetic Wound Healing by EGF Encapsulation in Gelatin-Alginate Coacervates. Pharmaceutics 2020, 12, 334. [Google Scholar] [CrossRef] [Green Version]
- Chouhan, D.; Mandal, B.B. Silk biomaterials in wound healing and skin regeneration therapeutics: From bench to bedside. Acta Biomater. 2019, 103, 24–51. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yan, L.; Yang, W.; Lan, Y.; Zhu, Q.; Xu, H.; Zheng, C.; Guo, R. Controlled-release neurotensin-loaded silk fibroin dressings improve wound healing in diabetic rat model. Bioact. Mater. 2019, 4, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Arango, M.C.; Montoya, Y.; Peresin, M.S.; Bustamante, J.; Álvarez-López, C. Silk sericin as a biomaterial for tissue engineering: A review. Int. J. Polym. Mater. Polym. Biomater. 2020, 70, 1115–1129. [Google Scholar] [CrossRef]
- Boonpavanitchakul, K.; Pimpha, N.; Kangwansupamonkon, W.; Magaraphan, R. Processing and antibacterial application of biodegradable sponge nano-composite materials of silver nanoparticles and silk sericin. Eur. Polym. J. 2020, 130, 109649. [Google Scholar] [CrossRef]
- Gilotra, S.; Chouhan, D.; Bhardwaj, N.; Nandi, S.K.; Mandal, B.B. Potential of silk sericin based nanofibrous mats for wound dressing applications. Mater. Sci. Eng. C 2018, 90, 420–432. [Google Scholar] [CrossRef]
- Park, M.; Shin, H.K.; Kim, B.-S.; Kim, M.J.; Kim, I.-S.; Park, B.-Y.; Kim, H.-Y. Effect of discarded keratin-based biocomposite hydrogels on the wound healing process in vivo. Mater. Sci. Eng. C 2015, 55, 88–94. [Google Scholar] [CrossRef]
- Rouse, J.G.; Van Dyke, M.E. A Review of Keratin-Based Biomaterials for Biomedical Applications. Materials 2010, 3, 999–1014. [Google Scholar] [CrossRef] [Green Version]
- Shankar, S.; Rhim, J.-W. Eco-friendly antimicrobial nanoparticles of keratin-metal ion complex. Mater. Sci. Eng. C 2019, 105, 110068. [Google Scholar] [CrossRef]
- Sharma, S.; Rostamabadi, H.; Gupta, S.; Nadda, A.K.; Kharazmi, M.S.; Jafari, S.M. Nano/micro-formulations of keratin in biocomposites, wound healing and drug delivery systems; recent advances in biomedical applications. Eur. Polym. J. 2022, 180, 111614. [Google Scholar] [CrossRef]
- Ye, J.-P.; Gong, J.-S.; Su, C.; Liu, Y.-G.; Jiang, M.; Pan, H.; Li, R.-Y.; Geng, Y.; Xu, Z.-H.; Shi, J.-S. Fabrication and characterization of high molecular keratin based nanofibrous membranes for wound healing. Colloids Surf. B Biointerfaces 2020, 194, 111158. [Google Scholar] [CrossRef]
- Kaczmarek-Szczepańska, B.; Miłek, O.; Michalska-Sionkowska, M.; Osyczka, A. Bio-studies of scaffolds based on chitosan/tannic acid cross-linked by glyoxal. Mater. Lett. 2021, 292, 129667. [Google Scholar] [CrossRef]
- Thao, N.T.; Wijerathna, H.; Kumar, R.S.; Choi, D.; Dananjaya, S.; Attanayake, A. Preparation and characterization of succinyl chitosan and succinyl chitosan nanoparticle film: In vitro and in vivo evaluation of wound healing activity. Int. J. Biol. Macromol. 2021, 193, 1823–1834. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.W.; Lim, H.; Na Chong, H.; Shim, W.S. Advances in Chitosan Material and its Hybrid Derivatives: A Review. Open Biomater. J. 2009, 1, 10–20. [Google Scholar] [CrossRef]
- Jayakumar, R.; Prabaharan, M.; Kumar, P.T.S.; Nair, S.V.; Tamura, H. Biomaterials based on chitin and chitosan in wound dressing applications. Biotechnol. Adv. 2011, 29, 322–337. [Google Scholar] [CrossRef]
- Hajiali, H.; Summa, M.; Russo, D.; Armirotti, A.; Brunetti, V.; Bertorelli, R.; Athanassiou, A.; Mele, E. Alginate–lavender nanofibers with antibacterial and anti-inflammatory activity to effectively promote burn healing. J. Mater. Chem. B 2016, 4, 1686–1695. [Google Scholar] [CrossRef] [Green Version]
- Zhai, Z.; Xu, K.; Mei, L.; Wu, C.; Liu, J.; Liu, Z.; Wan, L.; Zhong, W. Co-assembled supramolecular hydrogels of cell adhesive peptide and alginate for rapid hemostasis and efficacious wound healing. Soft Matter 2019, 15, 8603–8610. [Google Scholar] [CrossRef]
- Mndlovu, H.; du Toit, L.C.; Kumar, P.; Marimuthu, T.; Kondiah, P.P.; Choonara, Y.E.; Pillay, V. Development of a fluid-absorptive alginate-chitosan bioplatform for potential application as a wound dressing. Carbohydr. Polym. 2019, 222, 114988. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Raines, R.T. Collagen-based biomaterials for wound healing. Biopolymers 2014, 101, 821–833. [Google Scholar] [CrossRef] [Green Version]
- Bundjaja, V.; Santoso, S.P.; Angkawijaya, A.E.; Yuliana, M.; Soetaredjo, F.E.; Ismadji, S.; Ayucitra, A.; Gunarto, C.; Ju, Y.-H.; Ho, M.-H. Fabrication of cellulose carbamate hydrogel-dressing with rarasaponin surfactant for enhancing adsorption of silver nanoparticles and antibacterial activity. Mater. Sci. Eng. C 2020, 118, 111542. [Google Scholar] [CrossRef]
- Saravanakumar, K.; Park, S.; Santosh, S.S.; Ganeshalingam, A.; Thiripuranathar, G.; Sathiyaseelan, A.; Vijayasarathy, S.; Swaminathan, A.; Priya, V.V.; Wang, M.-H. Application of hyaluronic acid in tissue engineering, regenerative medicine, and nanomedicine: A review. Int. J. Biol. Macromol. 2022, 222, 2744–2760. [Google Scholar] [CrossRef]
- Mahzoon, S.; Siahaan, T.J.; Detamore, M.S. Functionalizing With Bioactive Peptides to Generate Bio-Instructive Scaffolds. In Bio-Instructive Scaffolds for Musculoskeletal Tissue Engineering and Regenerative Medicine; Brown, J.L., Kumar, S.G., Banik, B.L., Eds.; Academic Press: London, UK, 2017; pp. 37–52. [Google Scholar]
- Herlan, C.N.; Feser, D.; Schepers, U.; Bräse, S. Bio-instructive materials on-demand-combinatorial chemistry of peptoids, foldamers, and beyond. Chem. Commun. 2021, 57, 11131–11152. [Google Scholar] [CrossRef] [PubMed]
- Thapa, R.K.; Diep, D.B.; Tønnesen, H.H. Topical antimicrobial peptide formulations for wound healing: Current developments and future prospects. Acta Biomater. 2019, 103, 52–67. [Google Scholar] [CrossRef] [PubMed]
- de Souza, G.S.; Sonego, L.D.J.; Mundim, A.C.S.; Moraes, J.D.M.; Sales-Campos, H.; Lorenzón, E.N. Antimicrobial-wound healing peptides: Dual-function molecules for the treatment of skin injuries. Peptides 2022, 148, 170707. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.-J.; Sen, G.L.; Ward, N.L.; Johnston, A.; Chun, K.; Chen, Y.; Adase, C.; Sanford, J.A.; Gao, N.; Chensee, M.; et al. Antimicrobial Peptide LL37 and MAVS Signaling Drive Interferon-β Production by Epidermal Keratinocytes during Skin Injury. Immunity 2016, 45, 119–130. [Google Scholar] [CrossRef]
- Simonetti, O.; Cirioni, O.; Goteri, G.; Lucarini, G.; Kamysz, E.; Kamysz, W.; Orlando, F.; Rizzetto, G.; Molinelli, E.; Morroni, G.; et al. Efficacy of Cathelicidin LL-37 in an MRSA Wound Infection Mouse Model. Antibiotics 2021, 10, 1210. [Google Scholar] [CrossRef]
- Mahlapuu, M.; Björn, C.; Ekblom, J. Antimicrobial Peptides as Therapeutic Agents: Opportunities and Challenges. Crit Rev Biotechnol 2020, 40, 978–992. [Google Scholar] [CrossRef]
- Annabi, N.; Rana, D.; Sani, E.S.; Portillo-Lara, R.; Gifford, J.L.; Fares, M.M.; Mithieux, S.M.; Weiss, A.S. Engineering a sprayable and elastic hydrogel adhesive with antimicrobial properties for wound healing. Biomaterials 2017, 139, 229–243. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Fan, R.; Tong, A.; Yang, M.; Deng, J.; Zhou, L.; Zhang, X.; Guo, G. In situ gel-forming AP-57 peptide delivery system for cutaneous wound healing. Int. J. Pharm. 2015, 495, 560–571. [Google Scholar] [CrossRef]
- Wei, S.; Xu, P.; Yao, Z.; Cui, X.; Lei, X.; Li, L.; Dong, Y.; Zhu, W.; Guo, R.; Cheng, B. A composite hydrogel with co-delivery of antimicrobial peptides and platelet-rich plasma to enhance healing of infected wounds in diabetes. Acta Biomater. 2021, 124, 205–218. [Google Scholar] [CrossRef]
- Luo, T.; Kiick, K.L. Collagen-Like Peptide Bioconjugates. Bioconjugate Chem. 2017, 28, 816–827. [Google Scholar] [CrossRef]
- Zhang, Y.; Herling, M.; Chenoweth, D.M. General Solution for Stabilizing Triple Helical Collagen. J. Am. Chem. Soc. 2016, 138, 9751–9754. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Malamakal, R.M.; Chenoweth, D.M. Aza-Glycine Induces Collagen Hyperstability. J. Am. Chem. Soc. 2015, 137, 12422–12425. [Google Scholar] [CrossRef] [PubMed]
- Newberry, R.W.; VanVeller, B.; Raines, R.T. Thioamides in the collagen triple helix. Chem. Commun. 2015, 51, 9624–9627. [Google Scholar] [CrossRef] [Green Version]
- Luo, T.; Kiick, K.L. Collagen-like peptides and peptide–polymer conjugates in the design of assembled materials. Eur. Polym. J. 2013, 49, 2998–3009. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.Y.; Mo, X.; Chen, C.S.; Yu, S.M. Facile Modification of Collagen Directed by Collagen Mimetic Peptides. J. Am. Chem. Soc. 2005, 127, 4130–4131. [Google Scholar] [CrossRef]
- Parenteau-Bareil, R.; Gauvin, R.; Berthod, F. Collagen-Based Biomaterials for Tissue Engineering Applications. Materials 2010, 3, 1863–1887. [Google Scholar] [CrossRef] [Green Version]
- Wang, A.Y.; Leong, S.; Liang, Y.-C.; Huang, R.C.C.; Chen, C.; Yu, S.M. Immobilization of Growth Factors on Collagen Scaffolds Mediated by Polyanionic Collagen Mimetic Peptides and Its Effect on Endothelial Cell Morphogenesis. Biomacromolecules 2008, 9, 2929–2936. [Google Scholar] [CrossRef]
- Li, Y.; Ho, D.; Meng, H.; Chan, T.R.; An, B.; Yu, H.; Brodsky, B.; Jun, A.S.; Yu, S.M. Direct Detection of Collagenous Proteins by Fluorescently Labeled Collagen Mimetic Peptides. Bioconjugate Chem. 2012, 24, 9–16. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Foss, C.A.; Summerfield, D.D.; Doyle, J.J.; Torok, C.M.; Dietz, H.C.; Pomper, M.G.; Yu, S.M. Targeting collagen strands by photo-triggered triple-helix hybridization. Proc. Natl. Acad. Sci. USA 2012, 109, 14767–14772. [Google Scholar] [CrossRef] [Green Version]
- Chow, D.; Nunalee, M.L.; Lim, D.W.; Simnick, A.J.; Chilkoti, A. Peptide-based biopolymers in biomedicine and biotechnology. Mater. Sci. Eng. R: Rep. 2008, 62, 125–155. [Google Scholar] [CrossRef] [Green Version]
- Luo, T.; Kiick, K.L. Noncovalent Modulation of the Inverse Temperature Transition and Self-Assembly of Elastin-b-Collagen-like Peptide Bioconjugates. J. Am. Chem. Soc. 2015, 137, 15362–15365. [Google Scholar] [CrossRef] [Green Version]
- Chattopadhyay, S.; Guthrie, K.M.; Teixeira, L.; Murphy, C.J.; Dubielzig, R.R.; McAnulty, J.F.; Raines, R.T. Anchoring a cytoactive factor in a wound bed promotes healing. J. Tissue Eng. Regen. Med. 2014, 10, 1012–1020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, J.; Tong, Y.W. Self-Assembly of Collagen-Mimetic Peptide Amphiphiles into Biofunctional Nanofiber. ACS Nano 2011, 5, 7739–7747. [Google Scholar] [CrossRef] [PubMed]
- Krishna, O.D.; Jha, A.K.; Jia, X.; Kiick, K.L. Integrin-mediated adhesion and proliferation of human MSCs elicited by a hydroxyproline-lacking, collagen-like peptide. Biomaterials 2011, 32, 6412–6424. [Google Scholar] [CrossRef]
- Urello, M.A.; Kiick, K.L.; Sullivan, M.O. Integration of growth factor gene delivery with collagen-triggered wound repair cascades using collagen-mimetic peptides. Bioeng. Transl. Med. 2016, 1, 207–219. [Google Scholar] [CrossRef]
- Thapa, R.K.; Kiick, K.L.; Sullivan, M.O. Encapsulation of collagen mimetic peptide-tethered vancomycin liposomes in collagen-based scaffolds for infection control in wounds. Acta Biomater. 2019, 103, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Mathew-Steiner, S.; Roy, S.; Sen, C. Collagen in Wound Healing. Bioengineering 2021, 8, 63. [Google Scholar] [CrossRef]
- Kolácná, L.; Bakesová, J.; Varga, F.; Kostakova, E.K.; Plánka, L.; Necas, A.; Lukáš, D.; Amler, E.; Pelouch, V. Biochemical and biophysical aspects of collagen nanostructure in the extracellular matrix. Physiol. Res. 2007, 56, 51–60. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Theato, P. Collagen and collagen mimetic peptide conjugates in polymer science. Eur. Polym. J. 2013, 49, 2986–2997. [Google Scholar] [CrossRef]
- Xu, Y.; Kirchner, M. Collagen Mimetic Peptides. Bioengineering 2021, 8, 5. [Google Scholar] [CrossRef]
- Patil, V.A.; Masters, K.S. Engineered Collagen Matrices. Bioengineering 2020, 7, 163. [Google Scholar] [CrossRef] [PubMed]
- Berger, A.J.; Linsmeier, K.M.; Kreeger, P.K.; Masters, K.S. Decoupling the effects of stiffness and fiber density on cellular behaviors via an interpenetrating network of gelatin-methacrylate and collagen. Biomaterials 2017, 141, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, S.; Teixeira, L.B.C.; Kiessling, L.L.; McAnulty, J.F.; Raines, R.T. Bifunctional Peptide that Anneals to Damaged Collagen and Clusters TGF-β Receptors Enhances Wound Healing. ACS Chem. Biol. 2022, 17, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Heras, K.L.; Igartua, M.; Santos-Vizcaino, E.; Hernandez, R.M. Cell-based dressings: A journey through chronic wound management. Biomater. Adv. 2022, 135, 212738. [Google Scholar] [CrossRef]
- van de Vyver, M.; Idensohn, P.; Niesler, C. A regenerative approach to the pharmacological management of hard-to-heal wounds. Biochimie 2022, 194, 67–78. [Google Scholar] [CrossRef]
- Jackson, W.M.; Nesti, L.J.; Tuan, R.S. Concise Review: Clinical Translation of Wound Healing Therapies Based on Mesenchymal Stem Cells. STEM CELLS Transl. Med. 2011, 1, 44–50. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.-Z.; Gou, M.; Da, L.-C.; Zhang, M.W.-Q.; Xie, H.-Q. Mesenchymal Stem Cells for Chronic Wound Healing: Current Status of Preclinical and Clinical Studies. Tissue Eng. Part B Rev. 2020, 26, 555–570. [Google Scholar] [CrossRef]
- Nilforoushzadeh, M.A.; Yazdi, M.K.; Ghavami, S.B.; Farokhimanesh, S.; Amirabad, L.M.; Zarrintaj, P.; Saeb, M.R.; Hamblin, M.R.; Zare, M.; Mozafari, M. Mesenchymal Stem Cell Spheroids Embedded in an Injectable Thermosensitive Hydrogel: An In Situ Drug Formation Platform for Accelerated Wound Healing. ACS Biomater. Sci. Eng. 2020, 6, 5096–5109. [Google Scholar] [CrossRef]
- Ribeiro, J.; Pereira, T.; Amorim, I.; Caseiro, A.R.; Lopes, M.A.; Lima, J.F.T.F.; Gartner, A.; Santos, A.R.C.; Bartolo, P.; Rodrigues, J.M.; et al. Cell Therapy with Human MSCs Isolated from the Umbilical Cord Wharton Jelly Associated to a PVA Membrane in the Treatment of Chronic Skin Wounds. Int. J. Med Sci. 2014, 11, 979–987. [Google Scholar] [CrossRef] [Green Version]
- Navone, S.E.; Pascucci, L.; Dossena, M.; Ferri, A.; Invernici, G.; Acerbi, F.; Cristini, S.; Bedini, G.; Tosetti, V.; Ceserani, V.; et al. Decellularized silk fibroin scaffold primed with adipose mesenchymal stromal cells improves wound healing in diabetic mice. Stem Cell Res. Ther. 2014, 5, 7. [Google Scholar] [CrossRef] [Green Version]
- Kumar, L.K.; Kandoi, S.; Misra, R.; Vijayalakshmi, S.; Rajagopal, K.; Verma, R.S. The mesenchymal stem cell secretome: A new paradigm towards cell-free therapeutic mode in regenerative medicine. Cytokine Growth Factor Rev. 2019, 46, 1–9. [Google Scholar] [CrossRef]
- Moon, K.-C.; Suh, H.-S.; Kim, K.-B.; Han, S.-K.; Young, K.-W.; Lee, J.-W.; Kim, M.-H. Potential of Allogeneic Adipose-Derived Stem Cell–Hydrogel Complex for Treating Diabetic Foot Ulcers. Diabetes 2019, 68, 837–846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarasúa, J.G.; López, S.P.; Viejo, M.; Basterrechea, M.P.; Rodríguez, A.F.; Gutiérrez, A.F.; Gala, J.G.; Menéndez, Y.M.; Augusto, D.E.; Arias, A.P.; et al. Treatment of pressure ulcers with autologous bone marrow nuclear cells in patients with spinal cord injury. J. Spinal Cord Med. 2011, 34, 301–307. [Google Scholar] [CrossRef] [Green Version]
- Rasouli, M.; Rahimi, A.; Soleimani, M.; Keshel, S.H. The interplay between extracellular matrix and progenitor/stem cells during wound healing: Opportunities and future directions. Acta Histochem. 2021, 123, 151785. [Google Scholar] [CrossRef]
- Dash, B.C.; Xu, Z.; Lin, L.; Koo, A.; Ndon, S.U.; Berthiaume, F.; Dardik, A.; Hsia, H.C. Stem Cells and Engineered Scaffolds for Regenerative Wound Healing. Bioengineering 2018, 5, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, K.; Lawler, J. The interaction of Thrombospondins with extracellular matrix proteins. J. Cell Commun. Signal. 2009, 3, 177–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brizzi, M.F.; Tarone, G.; Defilippi, P. Extracellular matrix, integrins, and growth factors as tailors of the stem cell niche. Curr. Opin. Cell Biol. 2012, 24, 645–651. [Google Scholar] [CrossRef]
- Pardo-Saganta, A.; Calvo, I.A.; Saez, B.; Prosper, F. Role of the Extracellular Matrix in Stem Cell Maintenance. Curr. Stem Cell Rep. 2019, 5, 1–10. [Google Scholar] [CrossRef]
- Kucharzewski, M.; Rojczyk, E.; Wilemska-Kucharzewska, K.; Wilk, R.; Hudecki, J.; Los, M.J. Novel trends in application of stem cells in skin wound healing. Eur. J. Pharmacol. 2018, 843, 307–315. [Google Scholar] [CrossRef]
- Robb, K.P.; Shridhar, A.; Flynn, L.E. Decellularized Matrices As Cell-Instructive Scaffolds to Guide Tissue-Specific Regeneration. ACS Biomater. Sci. Eng. 2017, 4, 3627–3643. [Google Scholar] [CrossRef]
- Huang, S.-P.; Hsu, C.-C.; Chang, S.-C.; Wang, C.-H.; Deng, S.-C.; Dai, N.-T.; Chen, T.-M.; Chan, J.Y.-H.; Chen, S.-G.; Huang, S.-M. Adipose-Derived Stem Cells Seeded on Acellular Dermal Matrix Grafts Enhance Wound Healing in a Murine Model of a Full-Thickness Defect. Ann. Plast. Surg. 2012, 69, 656–662. [Google Scholar] [CrossRef] [PubMed]
- Sahin, I.; Ozturk, S.; Deveci, M.; Ural, A.U.; Onguru, O.; Isik, S. Experimental assessment of the neo-vascularisation of acellular dermal matrix in the wound bed pretreated with mesenchymal stem cell under subatmospheric pressure. J. Plast. Reconstr. Aesthetic Surg. 2014, 67, 107–114. [Google Scholar] [CrossRef]
- Everts, P.; Onishi, K.; Jayaram, P.; Lana, J.; Mautner, K. Platelet-Rich Plasma: New Performance Understandings and Therapeutic Considerations in 2020. Int. J. Mol. Sci. 2020, 21, 7794. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, P.; Law, J.X.; Ng, S.-F. Delivery systems for platelet derived growth factors in wound healing: A review of recent developments and global patent landscape. J. Drug Deliv. Sci. Technol. 2022, 71, 103270. [Google Scholar] [CrossRef]
- Metzemaekers, M.; Gouwy, M.; Proost, P. Neutrophil chemoattractant receptors in health and disease: Double-edged swords. Cell. Mol. Immunol. 2020, 17, 433–450. [Google Scholar] [CrossRef]
- Yamakawa, S.; Hayashida, K. Advances in surgical applications of growth factors for wound healing. Burn. Trauma 2019, 7, 10. [Google Scholar] [CrossRef] [Green Version]
- Eisinger, F.; Patzelt, J.; Langer, H.F. The Platelet Response to Tissue Injury. Front. Med. 2018, 5, 317. [Google Scholar] [CrossRef] [Green Version]
- Xue, M.; Jackson, C.J. Extracellular Matrix Reorganization During Wound Healing and Its Impact on Abnormal Scarring. Adv. Wound Care 2015, 4, 119–136. [Google Scholar] [CrossRef] [Green Version]
- Mantha, S.; Pillai, S.; Khayambashi, P.; Upadhyay, A.; Zhang, Y.; Tao, O.; Pham, H.M.; Tran, S.D. Smart Hydrogels in Tissue Engineering and Regenerative Medicine. Materials 2019, 12, 3323. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Yao, D.; Zhao, W.; Zhang, R.; Yu, B.; Ma, G.; Li, Y.; Hao, D.; Xu, F. Engineering Platelet-Rich Plasma Based Dual-Network Hydrogel as a Bioactive Wound Dressing with Potential Clinical Translational Value. Adv. Funct. Mater. 2020, 31, 2009258. [Google Scholar] [CrossRef]
- Liao, X.; Liang, J.-X.; Li, S.-H.; Huang, S.; Yan, J.-X.; Xiao, L.-L.; Song, J.-X.; Liu, H.-W. Allogeneic Platelet-Rich Plasma Therapy as an Effective and Safe Adjuvant Method for Chronic Wounds. J. Surg. Res. 2019, 246, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, M.H.; Molavi, B.; Mohammadi, S.; Nikbakht, M.; Mohammadi, A.M.; Mostafaei, S.; Norooznezhad, A.H.; Abdegah, A.G.; Ghavamzadeh, A. Evaluation of wound healing in diabetic foot ulcer using platelet-rich plasma gel: A single-arm clinical trial. Transfus. Apher. Sci. 2016, 56, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Kargozar, S.; Hamzehlou, S.; Baino, F. Can bioactive glasses be useful to accelerate the healing of epithelial tissues? Mater. Sci. Eng. C 2019, 97, 1009–1020. [Google Scholar] [CrossRef] [PubMed]
- Mehrabi, T.; Mesgar, A.S.; Mohammadi, Z. Bioactive Glasses: A Promising Therapeutic Ion Release Strategy for Enhancing Wound Healing. ACS Biomater. Sci. Eng. 2020, 6, 5399–5430. [Google Scholar] [CrossRef] [PubMed]
- Kargozar, S.; Baino, F.; Hamzehlou, S.; Hill, R.G.; Mozafari, M. Bioactive glasses entering the mainstream. Drug Discov. Today 2018, 23, 1700–1704. [Google Scholar] [CrossRef]
- Rahaman, M. Bioactive ceramics and glasses for tissue engineering. In Tissue Engineering Using Ceramics and Polymers; Woodhead Publishing: Sawston, UK, 2014; pp. 67–114. [Google Scholar] [CrossRef]
- Gorustovich, A.A.; Roether, J.A.; Boccaccini, A.R. Effect of Bioactive Glasses on Angiogenesis: A Review of In Vitro and In Vivo Evidences. Tissue Eng. Part B Rev. 2010, 16, 199–207. [Google Scholar] [CrossRef]
- Kargozar, S.; Baino, F.; Hamzehlou, S.; Hill, R.G.; Mozafari, M. Bioactive Glasses: Sprouting Angiogenesis in Tissue Engineering. Trends Biotechnol. 2018, 36, 430–444. [Google Scholar] [CrossRef]
- Zeng, Q.; Han, Y.; Li, H.; Chang, J. Design of a thermosensitive bioglass/agarose–alginate composite hydrogel for chronic wound healing. J. Mater. Chem. B 2015, 3, 8856–8864. [Google Scholar] [CrossRef]
- Zhou, Y.; Gao, L.; Peng, J.; Xing, M.; Han, Y.; Wang, X.; Xu, Y.; Chang, J. Bioglass Activated Albumin Hydrogels for Wound Healing. Adv. Heal. Mater. 2018, 7, e1800144. [Google Scholar] [CrossRef]
- Urello, M.A.; Kiick, K.L.; Sullivan, M.O. ECM turnover-stimulated gene delivery through collagen-mimetic peptide-plasmid integration in collagen. Acta Biomater. 2017, 62, 167–178. [Google Scholar] [CrossRef]
- Yao, B.; Wang, R.; Wang, Y.; Zhang, Y.; Hu, T.; Song, W.; Li, Z.; Huang, S.; Fu, X. Biochemical and structural cues of 3D-printed matrix synergistically direct MSC differentiation for functional sweat gland regeneration. Sci. Adv. 2020, 6, eaaz1094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, B.P.; Leong, K.W. Scaffolding in Tissue Engineering: General Approaches and Tissue-Specific Considerations. Eur. Spine J. 2008, 17, 467–479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Liu, Y.; Zhang, Y.; Yao, B.; Enhejirigala; Li, Z.; Song, W.; Wang, Y.; Duan, X.; Yuan, X.; et al. Biophysical and Biochemical Cues of Biomaterials Guide Mesenchymal Stem Cell Behaviors. Front. Cell Dev. Biol. 2021, 9, 640388. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Yu, M.; Lu, W.; Kong, J. Three-dimensional (3D), macroporous, elastic, and biodegradable nanocomposite scaffold for in situ bone regeneration: Toward structural, biophysical, and biochemical cues integration. Compos. Part B Eng. 2021, 225, 109270. [Google Scholar] [CrossRef]
- Man, W.; Yang, S.; Cao, Z.; Lu, J.; Kong, X.; Sun, X.; Zhao, L.; Guo, Y.; Yao, S.; Wang, G.; et al. A multi-modal delivery strategy for spinal cord regeneration using a composite hydrogel presenting biophysical and biochemical cues synergistically. Biomaterials 2021, 276, 120971. [Google Scholar] [CrossRef]









| Biopolymer | Composition | Biological Role in Wound Healing | Ref. |
|---|---|---|---|
| Chitosan | N-acetyl glucosamine linked by β-1, 4 glycosidic linkages | Haemostatic Induces fibroblast and keratinocytes migration and proliferation. | [28,30,74,75,76,77] |
| Alginate | 1, 4-linked β-d-mannuronic and α-L-guluronic residues | Haemostatic Exudate draining Stimulated monocytes, induces fibroblast proliferation and migration | [39,41,42,78,79,80] |
| Collagen | Amino acids linked by amide linkage | Influences blood clotting cascade Induces fibroblast proliferation, induces ECM components, chemotactic for macrophages | [60,81] |
| Cellulose | β-d-Glucose linked by β-1, 4-glycosidic linkage | Antibacterial Retention of moisture, absorption of exudates | [37,44,82] |
| Hyaluronic acid | D-glucuronic acid and N-acetyl-d-glucosamine linked by β-1, 4 and β-1, 3 glycosidic linkages | Stimulates fibroblasts and keratinocytes proliferation and migration, anti-inflammatory | [53,54,55,56,83] |
| Wound Dressing (with Application) | Biochemical Cue | Biological Performance | Ref. |
|---|---|---|---|
| In situ forming poly (L-lactic acid)-Pluronic L35 hydrogel loaded with antimicrobial peptides 57 nanoparticles (chronically infected wounds) | AMP nanoparticles | Promoted cutaneous wound healing by enhancing granulation tissue formation, increasing collagen deposition, and promoting angiogenesis | [92] |
| A composite hydrogel for the co-delivery of antimicrobial peptides and platelet-rich plasma (chronically infected wounds) | AMP with PRP | Improved wound healing in a diabetic mouse infection model by controlling inflammation, accelerating collagen deposition and angiogenesis | [93] |
| Collagen gels with CMP-immobilized or encapsulated DNA polyplexes (PDGF-BB delivery) (wound regeneration) | PEI DNA polyplexes with CMPs | Improved expression of PDGF-BB, proliferation, extracellular matrix production, and chemotaxis | [155] |
| Encapsulation of collagen mimetic peptide-tethered vancomycin liposomes in collagen-based scaffolds (chronic MRSA-infected wounds) | CMP | Sustained vancomycin release and enhanced in vitro and in vivo antibacterial properties against MRSA and closure rates | [110] |
| CMP-SubP conjugate (diabetic wounds) | CMP as a pylon with Substance P | CMP anneals to damaged collagen strands. Enhanced wound closure with noteworthy re-epithelialization and reduced inflammation in db/db mice | [106] |
| CMP-TGF-β-inducing peptide conjugate (severe wounds) | CMP as a pylon with peptide LTGKNFPMFHRN | Enhanced collagen deposition and wound closure in db/db mice by upregulation of the TGF-β signaling pathway | [117] |
| Mesenchymal stem cell spheroids embedded in an injectable thermosensitive semi-IPN hydrogel (cutaneous wounds) | MSCs | Faster wound closure in full-thickness wound models with reduced scarring. Well-organized collagen fibrils and high expression of the angiogenesis biomarker CD31 were also noted | [122] |
| Human MSCs in a PVA membrane (chronic wounds) | MSCs | Evaluated in dog non-healing skin lesions advancement in skin regeneration with a decreased expansion of ulcerated areas | [123] |
| Silk fibroin scaffold primed with adipose mesenchymal stromal cells (chronic diabetic ulcers) | MSCs | Improved tissue regeneration and reduction in wound region in db/db mice. Enhanced angiogenesis and matrix remodeling | [124] |
| Allogeneic Adipose-Derived Stem Cell-Hydrogel Complex (diabetic foot ulcers) | ASCs | Fifty-nine patients in a randomized clinical trial. Complete wound closure was achieved for 82% in the treatment group and 53% in the control group in week 12. | [126] |
| Autologous bone marrow nuclear cells (pressure ulcers) | BM-MNCs | In nineteen patients (86.36%), the pressure ulcers treated with BM-MNCs had fully healed after 21 days. Reduced hospital time, reduced treatment application time, and no reoccurrence of resolved ulcers was noted | [127] |
| Adipose-derived stem cells seeded on acellular dermal matrix grafts (full-thickness cutaneous wounds) | ASCs | Enhanced wound healing, angiogenesis, neo-vascularization, and VEGF-expressing ASCs were detected | [135] |
| Acellular dermal matrix with mesenchymal stem cell (full-thickness cutaneous wounds) | MSCs | Induced angiogenesis more efficiently than NPWT in rat models and improved neo-vascularization of the acellular dermal matrix | [136] |
| Platelet-Rich Plasma Based Dual-Network Hydrogel (various wound treatments) | PRP | In rats, the gel promoted rapid re-epithelialization, up-regulated growth factors, and early transitions in the wound healing and angiogenesis stages. It also exhibited superior healing efficiency in a porcine wound model. | [144] |
| Allogeneic Platelet-Rich Plasma Therapy (chronic wounds) | PRP | 60-patient randomized clinical trial showed improved chronic wound healing | [145] |
| Platelet-rich plasma gel (diabetic foot ulcers) | PRP | Longitudinal and single-arm trial of 100 patients. The wound area significantly decreased, and healing times were reduced to 8 weeks | [146] |
| Thermosensitive bioglass/agarose–alginate composite hydrogel (chronic wounds) | BG | Enhanced vasculature and epithelium formation in a rabbit ear ischemic wound model | [153] |
| Bioglass-activated albumin hydrogel (chronic wounds) | BG | In the full-thickness excisional chronic wound model in mice, the gel stimulated angiogenesis, neo-vascularization, and enhanced epithelium regeneration | [154] |
| Biochemical and structural cues of 3D-printed matrix (MSC-based therapies) | MSCs | Biochemical and structural cues of 3D-printed matrix synergistically directed MSC differentiation to functional sweat glands in vitro and in vivo | [156] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, V.; Marimuthu, T.; Makatini, M.M.; Choonara, Y.E. Biopolymer-Based Wound Dressings with Biochemical Cues for Cell-Instructive Wound Repair. Polymers 2022, 14, 5371. https://doi.org/10.3390/polym14245371
Singh V, Marimuthu T, Makatini MM, Choonara YE. Biopolymer-Based Wound Dressings with Biochemical Cues for Cell-Instructive Wound Repair. Polymers. 2022; 14(24):5371. https://doi.org/10.3390/polym14245371
Chicago/Turabian StyleSingh, Variksha, Thashree Marimuthu, Maya M. Makatini, and Yahya E. Choonara. 2022. "Biopolymer-Based Wound Dressings with Biochemical Cues for Cell-Instructive Wound Repair" Polymers 14, no. 24: 5371. https://doi.org/10.3390/polym14245371