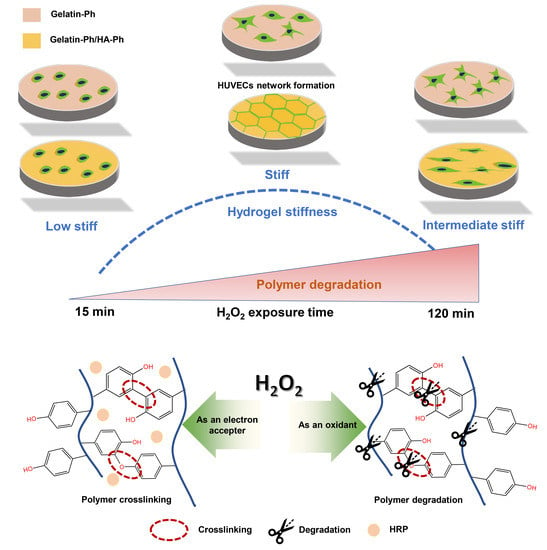
Human Umbilical Vein Endothelial Cells Form a Network on a Hyaluronic Acid/Gelatin Composite Hydrogel Moderately Crosslinked and Degraded by Hydrogen Peroxide
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Mechanical Property Measurement
2.3. Enzymatic Degradation
2.4. Molecular Weight Analysis
2.5. Scanning Electron Microscope Observation of Freeze-Dried Hydrogels
2.6. Cell Culture
2.7. Cell Viability and Adhesion
2.8. HUVECs Network Formation
2.9. Statistical Analysis
3. Results and Discussion
3.1. Properties of Gelatin-Ph/HA-Ph Hydrogels
3.2. HUVECs Behavior on Hydrogels
3.3. HUVECs Network Formation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brassard-Jollive, N.; Monnot, C.; Muller, L.; Germain, S. In Vitro 3D Systems to Model Tumor Angiogenesis and Interactions With Stromal Cells. Front. Cell Dev. Biol. 2020, 8, 1206. [Google Scholar] [CrossRef] [PubMed]
- Bogorad, M.I.; DeStefano, J.; Wong, A.D.; Searson, P.C. Tissue-Engineered 3D Microvessel and Capillary Network Models for the Study of Vascular Phenomena. Microcirculation 2017, 24, e12360. [Google Scholar] [CrossRef] [PubMed]
- Coelho-Santos, V.; Berthiaume, A.A.; Ornelas, S.; Stuhlmann, H.; Shih, A.Y. Imaging the Construction of Capillary Networks in the Neonatal Mouse Brain. Proc. Natl. Acad. Sci. USA 2021, 118, e2100866118. [Google Scholar] [CrossRef] [PubMed]
- Saunders, R.L.; Hammer, D.A. Assembly of Human Umbilical Vein Endothelial Cells on Compliant Hydrogels. Cell. Mol. Bioeng. 2010, 3, 60–67. [Google Scholar] [CrossRef] [Green Version]
- Yeon, J.H.; Ryu, H.R.; Chung, M.; Hu, Q.P.; Jeon, N.L. In Vitro Formation and Characterization of a Perfusable Three-Dimensional Tubular Capillary Network in Microfluidic Devices. Lab Chip 2012, 12, 2815–2822. [Google Scholar] [CrossRef]
- Arnaoutova, I.; Kleinman, H.K. In Vitro Angiogenesis: Endothelial Cell Tube Formation on Gelled Basement Membrane Extract. Nat. Protoc. 2010, 5, 628–635. [Google Scholar] [CrossRef] [PubMed]
- Arnaoutova, I.; George, J.; Kleinman, H.K.; Benton, G. The Endothelial Cell Tube Formation Assay on Basement Membrane Turns 20: State of the Science and the Art. Angiogenesis 2009, 12, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Chwalek, K.; Bray, L.J.; Werner, C. Tissue-Engineered 3D Tumor Angiogenesis Models: Potential Technologies for Anti-Cancer Drug Discovery. Adv. Drug Deliv. Rev. 2014, 79–80, 30–39. [Google Scholar] [CrossRef] [Green Version]
- Tsurkan, M.V.; Hauser, P.V.; Zieris, A.; Carvalhosa, R.; Bussolati, B.; Freudenberg, U.; Camussi, G.; Werner, C. Growth Factor Delivery from Hydrogel Particle Aggregates to Promote Tubular Regeneration after Acute Kidney Injury. J. Control. Release 2013, 167, 248–255. [Google Scholar] [CrossRef]
- Sun, M.; Chi, G.; Li, P.; Lv, S.; Xu, J.; Xu, Z.; Xia, Y.; Tan, Y.; Xu, J.; Li, L.; et al. Effects of Matrix Stiffness on the Morphology, Adhesion, Proliferation and Osteogenic Differentiation of Mesenchymal Stem Cells. Int. J. Med. Sci. 2018, 15, 257–268. [Google Scholar] [CrossRef]
- Ni, Y.; Chiang, M.Y.M. Cell Morphology and Migration Linked to Substrate Rigidity. Soft Matter 2007, 3, 1285–1292. [Google Scholar] [CrossRef] [PubMed]
- Tzoneva, R.; Uzunova, V.; Apostolova, S.; Krüger-Genge, A.; Neffe, A.T.; Jung, F.; Lendlein, A. Angiogenic Potential of Endothelial and Tumor Cells Seeded on Gelatin-Based Hydrogels in Response to Electrical Stimulations. Clin. Hemorheol. Microcirc. 2016, 64, 941–949. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sun, L.; Huan, Y.; Zhao, H.; Deng, J. Application of BFGF and BDNF to Improve Angiogenesis and Cardiac Function. J. Surg. Res. 2006, 136, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Yee, D.; Hanjaya-Putra, D.; Bose, V.; Luong, E.; Gerecht, S. Hyaluronic Acid Hydrogels Support Cord-Like Structures from Endothelial Colony-Forming Cells. Tissue Eng. Part A 2011, 17, 1351–1361. [Google Scholar] [CrossRef] [Green Version]
- Monteiro, N.; He, W.; Franca, C.M.; Athirasala, A.; Bertassoni, L.E. Engineering Microvascular Networks in LED Light-Cured Cell-Laden Hydrogels. ACS Biomater. Sci. Eng. 2018, 4, 2563–2570. [Google Scholar] [CrossRef] [PubMed]
- Frenkel, J.S. The Role of Hyaluronan in Wound Healing. Int. Wound J. 2014, 11, 159–163. [Google Scholar] [CrossRef]
- Schwertfeger, K.L.; Cowman, M.K.; Telmer, P.G.; Turley, E.A.; McCarthy, J.B. Hyaluronan, Inflammation, and Breast Cancer Progression. Front. Immunol. 2015, 6, 236. [Google Scholar] [CrossRef]
- Gao, F.; Yang, C.X.; Mo, W.; Liu, Y.W.; He, Y.Q. Hyaluronan Oligosaccharides Are Potential Stimulators to Angiogenesis via RHAMM Mediated Signal Pathway in Wound Healing. Clin. Investig. Med. 2008, 31, E106–E116. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Fu, C.; Zhang, Q.; He, C.; Zhang, F.; Wei, Q. The Role of CD44 in Pathological Angiogenesis. FASEB J. 2020, 34, 13125–13139. [Google Scholar] [CrossRef]
- Zhao, L.; Mitomo, H.; Zhai, M.; Yoshii, F.; Nagasawa, N.; Kume, T. Synthesis of Antibacterial PVA/CM-Chitosan Blend Hydrogels with Electron Beam Irradiation. Carbohydr. Polym. 2003, 53, 439–446. [Google Scholar] [CrossRef]
- Sabaa, M.W.; Mohamed, R.R.; Eltaweel, S.H.; Seoudi, R.S. Crosslinked Poly(Vinyl Alcohol)/Carboxymethyl Chitosan Hydrogels for Removal of Metal Ions and Dyestuff from Aqueous Solutions. J. Appl. Polym. Sci. 2012, 123, 3459–3469. [Google Scholar] [CrossRef]
- Dmitriev, I.; Kuryndin, I.; Bobrova, N.; Smirnov, M. Swelling Behavior and Network Characterization of Hydrogels from Linear Polyacrylamide Crosslinked with Glutaraldehyde. Mater. Today Commun. 2015, 4, 93–100. [Google Scholar] [CrossRef]
- Liu, X.; Jiang, Y.; He, H.; Ping, W. Hydrogen Peroxide-Induced Degradation of Type I Collagen Fibers of Tilapia Skin. Food Struct. 2014, 2, 41–48. [Google Scholar] [CrossRef]
- Chen, H.; Qin, J.; Hu, Y. Efficient Degradation of High-Molecular-Weight Hyaluronic Acid by a Combination of Ultrasound, Hydrogen Peroxide, and Copper Ion. Molecules 2019, 24, 617. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Xu, A.; Xie, H.; Yu, W.; Xie, W.; Ma, X. Preparation of Low Molecular Weight Alginate by Hydrogen Peroxide Depolymerization for Tissue Engineering. Carbohydr. Polym. 2010, 79, 660–664. [Google Scholar] [CrossRef]
- Mubarok, W.; Elvitigala, K.C.M.L.; Nakahata, M.; Kojima, M.; Sakai, S. Modulation of Cell-Cycle Progression by Hydrogen Peroxide-Mediated Cross-Linking and Degradation of Cell-Adhesive Hydrogels. Cells 2022, 11, 881. [Google Scholar] [CrossRef]
- Mubarok, W.; Qu, Y.; Sakai, S. Influence of Hydrogen Peroxide-Mediated Cross-Linking and Degradation on Cell-Adhesive Gelatin Hydrogels. ACS Appl. Bio Mater. 2021, 4, 4184–4190. [Google Scholar] [CrossRef]
- Mubarok, W.; Elvitigala, K.C.M.L.; Sakai, S. Tuning Myogenesis by Controlling Gelatin Hydrogel Properties through Hydrogen Peroxide-Mediated Cross-Linking and Degradation. Gels 2022, 8, 387. [Google Scholar] [CrossRef]
- Sakai, S.; Hirose, K.; Taguchi, K.; Ogushi, Y.; Kawakami, K. An Injectable, in Situ Enzymatically Gellable, Gelatin Derivative for Drug Delivery and Tissue Engineering. Biomaterials 2009, 30, 3371–3377. [Google Scholar] [CrossRef]
- Ho, M.H.; Kuo, P.Y.; Hsieh, H.J.; Hsien, T.Y.; Hou, L.T.; Lai, J.Y.; Wang, D.M. Preparation of Porous Scaffolds by Using Freeze-Extraction and Freeze-Gelation Methods. Biomaterials 2004, 25, 129–138. [Google Scholar] [CrossRef]
- Catelas, I.; Sese, N.; Wu, B.M.; Dunn, J.C.Y.; Helgerson, S.; Tawil, B. Human Mesenchymal Stem Cell Proliferation and Osteogenic Differentiation in Fibrin Gels in Vitro. Tissue Eng. 2006, 12, 2385–2396. [Google Scholar] [CrossRef]
- Agarwal, V.; Tjandra, E.S.; Iyer, K.S.; Humfrey, B.; Fear, M.; Wood, F.M.; Dunlop, S.; Raston, C.L. Evaluating the Effects of Nacre on Human Skin and Scar Cells in Culture. Toxicol. Res. 2014, 3, 223–227. [Google Scholar] [CrossRef] [Green Version]
- DeCicco-Skinner, K.L.; Henry, G.H.; Cataisson, C.; Tabib, T.; Gwilliam, J.C.; Watson, N.J.; Bullwinkle, E.M.; Falkenburg, L.; O’Neill, R.C.; Morin, A.; et al. Endothelial Cell Tube Formation Assay for the In Vitro Study of Angiogenesis. J. Vis. Exp. 2014, 91, e51312. [Google Scholar] [CrossRef] [PubMed]
- Ni, X.; Ke, F.; Xiao, M.; Wu, K.; Kuang, Y.; Corke, H.; Jiang, F. The Control of Ice Crystal Growth and Effect on Porous Structure of Konjac Glucomannan-Based Aerogels. Int. J. Biol. Macromol. 2016, 92, 1130–1135. [Google Scholar] [CrossRef] [PubMed]
- Grenier, J.; Duval, H.; Barou, F.; Lv, P.; David, B.; Letourneur, D. Mechanisms of Pore Formation in Hydrogel Scaffolds Textured by Freeze-Drying. Acta Biomater. 2019, 94, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Zmora, S.; Glicklis, R.; Cohen, S. Tailoring the Pore Architecture in 3-D Alginate Scaffolds by Controlling the Freezing Regime during Fabrication. Biomaterials 2002, 23, 4087–4094. [Google Scholar] [CrossRef]
- Pang, X.; Li, W.; Chang, L.; Gautrot, J.E.; Wang, W.; Azevedo, H.S. Hyaluronan (HA) Immobilized on Surfaces via Self-Assembled Monolayers of HA-Binding Peptide Modulates Endothelial Cell Spreading and Migration through Focal Adhesion. ACS Appl. Mater. Interfaces 2021, 13, 25792–25804. [Google Scholar] [CrossRef]
- Caon, I.; Bartolini, B.; Parnigoni, A.; Caravà, E.; Moretto, P.; Viola, M.; Karousou, E.; Vigetti, D.; Passi, A. Revisiting the Hallmarks of Cancer: The Role of Hyaluronan. Semin. Cancer Biol. 2020, 62, 9–19. [Google Scholar] [CrossRef]
- Savani, R.C.; Cao, G.; Pooler, P.M.; Zaman, A.; Zhou, Z.; DeLisser, H.M. Differential Involvement of the Hyaluronan (HA) Receptors CD44 and Receptor for HA-Mediated Motility in Endothelial Cell Function and Angiogenesis. J. Biol. Chem. 2001, 276, 36770–36778. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.Z.; Cao, M.L.; Liu, Y.W.; He, Y.Q.; Yang, C.X.; Gao, F. CD44 Mediates Oligosaccharides of Hyaluronan-Induced Proliferation, Tube Formation and Signal Transduction in Endothelial Cells. Exp. Biol. Med. 2011, 236, 84–90. [Google Scholar] [CrossRef]
- Sabine, M.-N.; John, G.; Shant, K.; Msrk, S. Oligosaccharides of Hyaluronan Induce Angiogenesis through Distinct CD44 and RHAMM-Mediated Signalling Pathways Involving Cdc2 and Gamma-Adducin. Int. J. Oncol. 2009, 35, 761–773. [Google Scholar] [CrossRef] [Green Version]
- Park, D.; Kim, Y.; Kim, H.; Kim, K.; Lee, Y.S.; Choe, J.; Hahn, J.H.; Lee, H.; Jeon, J.; Choi, C.; et al. Hyaluronic Acid Promotes Angiogenesis by Inducing RHAMM-TGFβ Receptor Interaction via CD44-PKCδ. Mol. Cells 2012, 33, 563–574. [Google Scholar] [CrossRef] [PubMed]
- Califano, J.P.; Reinhart-King, C.A. A Balance of Substrate Mechanics and Matrix Chemistry Regulates Endothelial Cell Network Assembly. Cell. Mol. Bioeng. 2008, 1, 122–132. [Google Scholar] [CrossRef]
- Petrey, A.C.; de la Motte, C.A. Hyaluronan, a Crucial Regulator of Inflammation. Front. Immunol. 2014, 5, 101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tammi, M.I.; Oikari, S.; Pasonen-Seppänen, S.; Rilla, K.; Auvinen, P.; Tammi, R.H. Activated Hyaluronan Metabolism in the Tumor Matrix—Causes and Consequences. Matrix Biol. 2019, 78–79, 147–164. [Google Scholar] [CrossRef] [PubMed]







| Abbreviation | Hydrogel Composition | H2O2 Exposure Time (min) |
|---|---|---|
| G3-15 | 3 wt% Gelatin-Ph | 15 |
| G3-60 | 3 wt% Gelatin-Ph | 60 |
| G3-120 | 3 wt% Gelatin-Ph | 120 |
| G3/HA0.5-15 | 3 wt% Gelatin-Ph/0.5 wt% HA-Ph | 15 |
| G3/HA0.5-60 | 3 wt% Gelatin-Ph/0.5 wt% HA-Ph | 60 |
| G3/HA0.5-120 | 3 wt% Gelatin-Ph/0.5 wt% HA-Ph | 120 |
| G3.25-60 | 3.25 wt% Gelatin-Ph | 60 |
| G3/Alg0.5-60 | 3 wt% Gelatin-Ph/0.5 wt% Alginate-Ph | 60 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elvitigala, K.C.M.L.; Mubarok, W.; Sakai, S. Human Umbilical Vein Endothelial Cells Form a Network on a Hyaluronic Acid/Gelatin Composite Hydrogel Moderately Crosslinked and Degraded by Hydrogen Peroxide. Polymers 2022, 14, 5034. https://doi.org/10.3390/polym14225034
Elvitigala KCML, Mubarok W, Sakai S. Human Umbilical Vein Endothelial Cells Form a Network on a Hyaluronic Acid/Gelatin Composite Hydrogel Moderately Crosslinked and Degraded by Hydrogen Peroxide. Polymers. 2022; 14(22):5034. https://doi.org/10.3390/polym14225034
Chicago/Turabian StyleElvitigala, Kelum Chamara Manoj Lakmal, Wildan Mubarok, and Shinji Sakai. 2022. "Human Umbilical Vein Endothelial Cells Form a Network on a Hyaluronic Acid/Gelatin Composite Hydrogel Moderately Crosslinked and Degraded by Hydrogen Peroxide" Polymers 14, no. 22: 5034. https://doi.org/10.3390/polym14225034