Poly(Glycerol Succinate) as Coating Material for 1393 Bioactive Glass Porous Scaffolds for Tissue Engineering Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
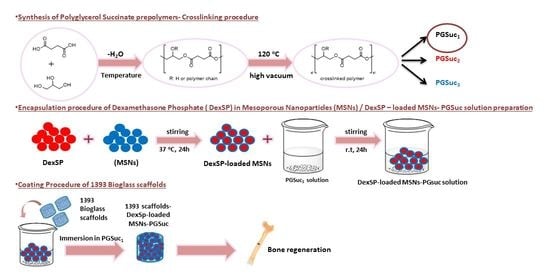
2.2. Synthesis of PGSuc
2.3. Characterization of Polymer
2.3.1. Nuclear Magnetic Resonance Spectroscopy
2.3.2. Fourier Transform Infrared Spectroscopy
2.3.3. X-ray Diffraction
2.3.4. Acid Value Determination
2.3.5. Hydrolysis Test
2.4. Synthesis of 1393 Bioactive Glass Scaffolds
2.5. Synthesis of MSNs
2.6. Drug Loading and Release of MSNs
2.7. Coating of Scaffolds
2.7.1. Coating with PGSu
2.7.2. Coating with PGSu + DexSP-laden MSNs
2.8. Characterization of Scaffolds
2.8.1. Scanning Electron Microscopy (SEM)
2.8.2. FTIR Analysis of Scaffolds
2.8.3. Mechanical Properties
2.8.4. Apatite-Forming Ability in SBF
2.8.5. Degradation
2.8.6. Cell Culture
2.8.7. Biocompatibility
2.8.8. Osteogenic Differentiation
- Alizarin red staining (ARS)
- Quantitative measurement of ALP activity and Reactive Oxygen Species (ROS) levels
3. Results
3.1. Synthesis and Characterization of PGSuc
3.1.1. 1H- and 13C-NMR Analysis
3.1.2. FTIR Spectroscopy
3.1.3. Hydrolysis Test
3.2. SEM Analysis of the Scaffolds
3.3. FTIR Analysis of MSNs and Scaffolds
3.4. X-ray Analysis of MSNs and Scaffolds
3.5. Degradation Study
3.6. Mechanical Properties of Scaffolds
3.7. Apatite-Forming Ability in SBF
3.7.1. Fourier Transform Infrared Spectroscopy (FTIR)
3.7.2. Scanning Electron Microscopy (SEM)
3.8. Drug Loading and Release Studies
3.9. Biocompatibility
3.10. Osteogenic Differentiation
3.10.1. Alizarin Red Staining (ARS)
3.10.2. Alkaline Phosphatase Activity (ALP)
3.10.3. Reactive Oxygen Species (ROS) Levels
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tsujimoto, T.; Uyama, H.; Kobayashi, S. Enzymatic synthesis and curing of biodegradable crosslinkable polyesters. Macromol. Biosci. 2002, 2, 329–335. [Google Scholar] [CrossRef]
- Naoloua, T.; Jbeily, M.; Scholtysek, P.; Kressler, J. Synthesis and characterization of stearoyl modified poly (glycerol adipate) containing ATRP initiator on its backbone. Adv. Mater. Res. 2013, 812, 1–11. [Google Scholar]
- Domínguez-Robles, J.; Larrañeta, E.; Fong, M.L.; Martin, N.K.; Irwin, N.J.; Mutjé, P.; Tarrés, Q.; Delgado-Aguilar, M. Lignin/poly(butylene succinate) composites with antioxidant and antibacterial properties for potential biomedical applications. Int. J. Biol. Macromol. 2020, 145, 92–99. [Google Scholar] [CrossRef]
- Zhang, H.; Grinstaff, M.W. Recent advances in glycerol polymers: Chemistry and biomedical applications. Macromol. Rapid Commun. 2014, 35, 1906–1924. [Google Scholar] [CrossRef]
- Taresco, V.; Creasey, R.G.; Kennon, J.; Mantovani, G.; Alexander, C.; Burley, J.C.; Garnett, M.C. Variation in structure and properties of poly(glycerol adipate) via control of chain branching during enzymatic synthesis. Polymer 2016, 89, 41–49. [Google Scholar] [CrossRef]
- Balla, E.; Daniilidis, V.; Karlioti, G.; Kalamas, T.; Stefanidou, M.; Bikiaris, N.D.; Vlachopoulos, A.; Koumentakou, I.; Bikiaris, D.N. Poly(lactic acid): A versatile biobased polymer for the future with multifunctional properties-from monomer synthesis, polymerization techniques and molecular weight increase to PLA applications. Polymers 2021, 13, 1822. [Google Scholar] [CrossRef] [PubMed]
- Vlachopoulos, A.; Karlioti, G.; Balla, E.; Daniilidis, V.; Kalamas, T.; Stefanidou, M.; Bikiaris, N.D.; Christodoulou, E.; Koumentakou, I.; Karavas, E.; et al. Poly(Lactic Acid)-Based Microparticles for Drug Delivery Applications: An Overview of Recent Advances. Pharmaceutics 2022, 14, 359. [Google Scholar] [CrossRef]
- Taresco, V.; Suksiriworapong, J.; Creasey, R.; Burley, J.C.; Mantovani, G.; Alexander, C.; Treacher, K.; Booth, J.; Garnett, M.C. Properties of acyl modified poly(glycerol-adipate) comb-like polymers and their self-assembly into nanoparticles. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 3267–3278. [Google Scholar] [CrossRef] [Green Version]
- Ionescu, M.; Petrović, Z.S. On the Mechanism of Base-Catalyzed Glycerol Polymerization and Copolymerization. Eur. J. Lipid Sci. Technol. 2018, 120, e1800004. [Google Scholar] [CrossRef]
- Gheybi, H.; Sattari, S.; Bodaghi, A.; Soleimani, K.; Dadkhah, A.; Adeli, M. Polyglycerols. Eng. Biomater. Drug Deliv. Syst. Beyond Polyethyl. Glycol 2018, 4, 103–171. [Google Scholar]
- Zamboulis, A.; Nakiou, E.A.; Christodoulou, E.; Bikiaris, D.N.; Kontonasaki, E.; Liverani, L.; Boccaccini, A.R. Polyglycerol hyperbranched polyesters: Synthesis, properties and pharmaceutical and biomedical applications. Int. J. Mol. Sci. 2019, 20, 6210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luman, N.R.; Kim, T.; Grinstaff, M.W. Dendritic polymers composed of glycerol and succinic acid: Synthetic methodologies and medical applications. Pure Appl. Chem. 2004, 76, 1375–1385. [Google Scholar] [CrossRef]
- Agach, M.; Delbaere, S.; Marinkovic, S.; Estrine, B.; Nardello-Rataj, V. Characterization, stability and ecotoxic properties of readily biodegradable branched oligoesters based on bio-sourced succinic acid and glycerol. Polym. Degrad. Stab. 2012, 97, 1956–1963. [Google Scholar] [CrossRef]
- Carnahan, M.A.; Middleton, C.; Kim, J.; Kim, T.; Grinstaff, M.W. Hybrid dendritic-linear polyester-ethers for in situ photopolymerization. J. Am. Chem. Soc. 2002, 124, 5291–5293. [Google Scholar] [CrossRef] [PubMed]
- Valerio, O.; Horvath, T.; Pond, C.; Misra, M.; Mohanty, A. Improved utilization of crude glycerol from biodiesel industries: Synthesis and characterization of sustainable biobased polyesters. Ind. Crops Prod. 2015, 78, 141–147. [Google Scholar] [CrossRef] [Green Version]
- Hoppe, A.; Jokic, B.; Janackovic, D.; Fey, T.; Greil, P.; Romeis, S.; Schmidt, J.; Peukert, W.; Lao, J.; Jallot, E.; et al. Cobalt-releasing 1393 bioactive glass-derived scaffolds for bone tissue engineering applications. ACS Appl. Mater. Interfaces 2014, 6, 2865–2877. [Google Scholar] [CrossRef]
- Fu, Q.; Rahaman, M.N.; Sonny Bal, B.; Brown, R.F.; Day, D.E. Mechanical and in vitro performance of 13-93 bioactive glass scaffolds prepared by a polymer foam replication technique. Acta Biomater. 2008, 4, 1854–1864. [Google Scholar] [CrossRef]
- Fiume, E.; Ciavattini, S.; Verné, E.; Baino, F. Foam replica method in the manufacturing of bioactive glass scaffolds: Out-of-date technology or still underexploited potential? Materials 2021, 14, 2795. [Google Scholar] [CrossRef]
- Smith, J.R.; Lamprou, D.A.; Larson, C.; Upson, S.J. Biomedical applications of polymer and ceramic coatings: A review of recent developments. Trans. Inst. Met. Finish. 2022, 100, 25–35. [Google Scholar] [CrossRef]
- Boffito, M.; Servello, L.; Arango-Ospina, M.; Miglietta, S.; Tortorici, M.; Sartori, S.; Ciardelli, G.; Boccaccini, A.R. Custom-Made Poly(urethane) Coatings Improve the Mechanical Properties of Bioactive Glass Scaffolds Designed for Bone Tissue Engineering. Polymers 2022, 14, 151. [Google Scholar] [CrossRef]
- Olivieri, F.; Castaldo, R.; Cocca, M.; Gentile, G.; Lavorgna, M. Mesoporous silica nanoparticles as carriers of active agents for smart anticorrosive organic coatings: A critical review. Nanoscale 2021, 13, 9091–9111. [Google Scholar] [CrossRef]
- Abaza, S.F.; Elbialy, N.S.; Mohamed, N. Incorporating silver nanoshell-coated mesoporous silica nanoparticles improves physicochemical and antimicrobial properties of chitosan films. Int. J. Biol. Macromol. 2021, 189, 792–801. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Liu, H.; Deng, H.; Xiao, L.; Qin, C.; Du, Y.; Shi, X. A study of chitosan hydrogel with embedded mesoporous silica nanoparticles loaded by ibuprofen as a dual stimuli-responsive drug release system for surface coating of titanium implants. Colloids Surf. B Biointerfaces 2014, 123, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Pouroutzidou, G.K.; Liverani, L.; Theocharidou, A.; Tsamesidis, I.; Lazaridou, M.; Christodoulou, E.; Beketova, A.; Pappa, C.; Triantafyllidis, K.S.; Anastasiou, A.D.; et al. Synthesis and characterization of mesoporous mg-and sr-doped nanoparticles for moxifloxacin drug delivery in promising tissue engineering applications. Int. J. Mol. Sci. 2021, 22, 577. [Google Scholar] [CrossRef]
- Gkiliopoulos, D.; Tsamesidis, I.; Theocharidou, A.; Pouroutzidou, G.K.; Christodoulou, E.; Stalika, E.; Xanthopoulos, K.; Bikiaris, D.; Triantafyllidis, K.; Kontonasaki, E. SBA-15 Mesoporous Silica as Delivery Vehicle for rhBMP-2 Bone Morphogenic Protein for Dental Applications. Nanomaterials 2022, 12, 822. [Google Scholar] [CrossRef]
- Cao, Y.; Xu, C.; Wright, P.P.; Liu, J.; Kong, Y.; Wang, Y.; Huang, X.; Song, H.; Fu, J.; Gao, F.; et al. Calcium-Doped Silica Nanoparticles Mixed with Phosphate-Doped Silica Nanoparticles for Rapid and Stable Occlusion of Dentin Tubules. ACS Appl. Nano Mater. 2021, 4, 8761–8769. [Google Scholar] [CrossRef]
- Helander, E.M.; Menard, B.L.; Harmon, C.M.; Homra, B.K.; Allain, A.V.; Bordelon, G.J.; Wyche, M.Q.; Padnos, I.W.; Lavrova, A.; Kaye, A.D. Multimodal Analgesia, Current Concepts, and Acute Pain Considerations. Curr. Pain Headache Rep. 2017, 21, 3. [Google Scholar] [CrossRef]
- Kragballe, K. Topical corticosteroids: Mechanisms of action. Acta Derm.-Venereol. Suppl. 1989, 69, 7–10. [Google Scholar]
- Chen, Q.Z.; Thompson, I.D.; Boccaccini, A.R. 45S5 Bioglass®-derived glass-ceramic scaffolds for bone tissue engineering. Biomaterials 2006, 27, 2414–2425. [Google Scholar] [CrossRef]
- Pouroutzidou, G.K.; Lazaridou, M.; Papoulia, C.; Tsamesidis, I.; Chrissafis, K.; Vourlias, G.; Paraskevopoulos, K.M.; Bikiaris, D.; Kontonasaki, E. Electrospun PLGA Membranes with Incorporated Moxifloxacin-Loaded Silica-Based Mesoporous Nanocarriers for Periodontal Regeneration. Nanomaterials 2022, 12, 850. [Google Scholar] [CrossRef]
- Karava, A.; Lazaridou, M.; Nanaki, S.; Michailidou, G.; Christodoulou, E.; Kostoglou, M.; Iatrou, H.; Bikiaris, D.N. Chitosan derivatives with mucoadhesive and antimicrobial properties for simultaneous nanoencapsulation and extended ocular release formulations of dexamethasone and chloramphenicol drugs. Pharmaceutics 2020, 12, 594. [Google Scholar] [CrossRef] [PubMed]
- Kokubo, T.; Kushitani, H.; Sakka, S.; Kitsugi, T.; Yamamuro, T. Solutions able to reproduce in vivo surface-structure changes in bioactive glass-ceramic A-W3. J. Biomed. Mater. Res. 1990, 24, 721–734. [Google Scholar] [CrossRef] [PubMed]
- Griffin, M.; Naderi, N.; Kalaskar, D.M.; Malins, E.; Becer, R.; Thornton, C.A.; Whitaker, I.S.; Mosahebi, A.; Butler, P.E.M.; Seifalian, A.M. Evaluation of Sterilisation Techniques for Regenerative Medicine Scaffolds Fabricated with Polyurethane Nonbiodegradable and Bioabsorbable Nanocomposite Materials. Int. J. Biomater. 2018, 2018, 6565783. [Google Scholar] [CrossRef]
- Filgueiras, M.R.T.; La Torre, G.; Hench, L.L. Solution effects on the surface reactions of three bioactive glass compositions. J. Biomed. Mater. Res. 1993, 27, 1485–1493. [Google Scholar] [CrossRef]
- Filippousi, M.; Siafaka, P.I.; Amanatiadou, E.P.; Nanaki, S.G.; Nerantzaki, M.; Bikiaris, D.N.; Vizirianakis, I.S.; Van Tendeloo, G. Modified chitosan coated mesoporous strontium hydroxyapatite nanorods as drug carriers. J. Mater. Chem. B 2015, 3, 5991–6000. [Google Scholar] [CrossRef]
- Li, P.; Ohtsuki, C.; Kokubo, T.; Nakanishi, K.; Soga, N.; de Groot, K. The role of hydrated silica, titania, and alumina in inducing apatite on implants. J. Biomed. Mater. Res. 1994, 28, 7–15. [Google Scholar] [CrossRef]
- Oliveira, J.M.; Correia, R.N.; Fernandes, M.H. Effects of Si speciation on the in vitro bioactivity of glasses. Biomaterials 2002, 23, 371–379. [Google Scholar] [CrossRef]
- Souza, M.T.; Crovace, M.C.; Schröder, C.; Eckert, H.; Peitl, O.; Zanotto, E.D. Effect of magnesium ion incorporation on the thermal stability, dissolution behavior and bioactivity in Bioglass-derived glasses. J. Non. Cryst. Solids 2013, 382, 57–65. [Google Scholar] [CrossRef]
- Maçon, A.L.B.; Kim, T.B.; Valliant, E.M.; Goetschius, K.; Brow, R.K.; Day, D.E.; Hoppe, A.; Boccaccini, A.R.; Kim, I.Y.; Ohtsuki, C.; et al. A unified in vitro evaluation for apatite-forming ability of bioactive glasses and their variants. J. Mater. Sci. Mater. Med. 2015, 26, 115. [Google Scholar] [CrossRef] [Green Version]
- Raynaud, S.; Champion, E.; Bernache-Assollant, D.; Thomas, P. Calcium phosphate apatites with variable Ca/P atomic ratio I. Synthesis, characterisation and thermal stability of powders. Biomaterials 2002, 23, 1065–1072. [Google Scholar] [CrossRef]
- Kontonasaki, E.; Zorba, T.; Papadopoulou, L.; Pavlidou, E.; Chatzistavrou, X.; Paraskevopoulos, K.; Koidis, P. Hydroxy carbonate apatite formation on particulate bioglass in vitro as a function of time. Cryst. Res. Technol. 2002, 37, 1165–1171. [Google Scholar] [CrossRef]
- Müller, L.; Müller, F.A. Preparation of SBF with different HCO3- content and its influence on the composition of biomimetic apatites. Acta Biomater. 2006, 2, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Ślósarczyk, A.; Paszkiewicz, Z.; Paluszkiewicz, C. FTIR and XRD evaluation of carbonated hydroxyapatite powders synthesized by wet methods. J. Mol. Struct. 2005, 744–747, 657–661. [Google Scholar] [CrossRef]
- Gerhardt, L.-C.; Boccaccini, A.R. Bioactive Glass and Glass-Ceramic Scaffolds for Bone Tissue Engineering. Materials 2010, 3, 3867–3910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larsen, E.; Røhl, L.; Linde, F.; Odgaard, A.; Jørgensen, J. Tensile and compressive properties of cancellous bone. J. Biomech. 1991, 24, 459. [Google Scholar] [CrossRef]
- Ali, A.; Ershad, M.; Vyas, V.K.; Hira, S.K.; Manna, P.P.; Singh, B.N.; Yadav, S.; Srivastava, P.; Singh, S.P.; Pyare, R. Studies on effect of CuO addition on mechanical properties and in vitro cytocompatibility in 1393 bioactive glass scaffold. Mater. Sci. Eng. C 2018, 93, 341–355. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Singh, B.N.; Hira, S.K.; Singh, S.P.; Pyare, R. ZnO modified 1393 bioactive scaffolds with enhanced cytocompatibility and mechanical performance. Ceram. Int. 2020, 46, 6703–6713. [Google Scholar] [CrossRef]
- Ali, A.; Singh, S.P.; Pyare, R. SrO assisted 1393 glass scaffold with enhanced biological compatibility. J. Non. Cryst. Solids 2020, 550, 120392. [Google Scholar] [CrossRef]
- Ali, A.; Singh, B.N.; Yadav, S.; Ershad, M.; Singh, S.K.; Mallick, S.P.; Pyare, R. CuO assisted borate 1393B3 glass scaffold with enhanced mechanical performance and cytocompatibility: An In vitro study. J. Mech. Behav. Biomed. Mater. 2021, 114, 104231. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Rahaman, M.N.; Day, D.E. Accelerated conversion of silicate bioactive glass (13–93) to hydroxyapatite in aqueous phosphate solution containing polyanions. J. Am. Ceram. Soc. 2009, 92, 2870–2876. [Google Scholar] [CrossRef]
- Choi, E.; Kim, S. Surface pH buffering to promote degradation of mesoporous silica nanoparticles under a physiological condition. J. Colloid Interface Sci. 2019, 533, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Faqhiri, H.; Hannula, M.; Kellomäki, M.; Calejo, M.T.; Massera, J. Effect of Melt-Derived Bioactive Glass Particles on the Properties of Chitosan Scaffolds. J. Funct. Biomater. 2019, 10, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seré, S.; De Roo, B.; Vervaele, M.; Van Gool, S.; Jacobs, S.; Seo, J.W.; Locquet, J.P. Altering the Biodegradation of Mesoporous Silica Nanoparticles by Means of Experimental Parameters and Surface Functionalization. J. Nanomater. 2018, 2018, 7390618. [Google Scholar] [CrossRef]
- Moghanian, A.; Ghorbanoghli, A.; Kazem-Rostami, M.; Pazhouheshgar, A.; Salari, E.; Saghafi Yazdi, M.; Alimardani, T.; Jahani, H.; Sharifian Jazi, F.; Tahriri, M. Novel antibacterial Cu/Mg-substituted 58S-bioglass: Synthesis, characterization and investigation of in vitro bioactivity. Int. J. Appl. Glas. Sci. 2020, 11, 685–698. [Google Scholar] [CrossRef]
- Shuai, C.; Guo, W.; Gao, C.; Yang, Y.; Xu, Y.; Liu, L.; Qin, T.; Sun, H.; Yang, S.; Feng, P.; et al. Calcium silicate improved bioactivity and mechanical properties of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) scaffolds. Polymers 2017, 9, 175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trzeciak, K.; Chotera-ouda, A.; Bak-sypien, I.I.; Potrzebowski, M.J. Mesoporous silica particles as drug delivery systems—The state of the art in loading methods and the recent progress in analytical techniques for monitoring these processes. Pharmaceutics 2021, 13, 950. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Chen, S.; Jin, Y.; Zhang, C.; Yang, X.; Ge, K.; Liang, X.J.; Li, Z.; Zhang, J. A traceable and bone-targeted nanoassembly based on defect-related luminescent mesoporous silica for enhanced osteogenic differentiation. J. Mater. Chem. B 2017, 5, 1585–1593. [Google Scholar] [CrossRef] [PubMed]
- Sordi, M.B.; Curtarelli, R.B.; da Silva, I.T.; Fongaro, G.; Benfatti, C.A.M.; de Souza Magini, R.; Cabral da Cruz, A.C. Effect of dexamethasone as osteogenic supplementation in in vitro osteogenic differentiation of stem cells from human exfoliated deciduous teeth. J. Mater. Sci. Mater. Med. 2021, 32, 1. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Pang, B.; Li, Y.; Zhu, D.; Pang, T.; Liu, Y. Dexamethasone has variable effects on mesenchymal stromal cells. Cytotherapy 2012, 14, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, R.S. Glucocorticoids, Osteocytes, and Skeletal Fragility: The Role of Bone Vascularity. Bone 2010, 46, 564–570. [Google Scholar] [CrossRef] [Green Version]
- Weinstein, R.S. Glucocorticoid-induced osteoporosis and osteonecrosis. Endocrinol. Metab. Clin. N. Am. 2012, 41, 595–611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Libánská, A.; Randárová, E.; Lager, F.; Renault, G.; Scherman, D.; Etrych, T. Polymer nanomedicines with Ph-sensitive release of dexamethasone for the localized treatment of inflammation. Pharmaceutics 2020, 12, 700. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, W. Adipose-derived stem cells: Implications in tissue regeneration. World J. Stem Cells 2014, 6, 312. [Google Scholar] [CrossRef] [PubMed]
- Anselme, K. Osteoblast adhesion on biomaterials. Biomaterials 2000, 21, 667–681. [Google Scholar] [CrossRef]
- Jafari, A.; Fakhri, V.; Kamrani, S.; Reza Ghaffarian Anbaran, S.; Su, C.H.; Goodarzi, V.; Pirouzfar, V.; Ali Khonakdar, H. Developemt of Flexible Nanocomposites Based on Poly(ε-caprolactone) for Tissue Engineering Application: The Contributing Role of Poly(glycerol succinic acid) and Polypyrrol. Eur. Polym. J. 2022, 164, 110984. [Google Scholar] [CrossRef]
- Kolbuk, D.; Jeznach, O.; Wrzecionek, M.; Gadomska-Gajadhur, A. Poly(glycerol succinate) as an eco-friendly component of PLLA and PLCL fibres towards medical applications. Polymers 2020, 12, 1731. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.R. Review of bioactive glass: From Hench to hybrids. Acta Biomater. 2013, 9, 4457–4486. [Google Scholar] [CrossRef] [PubMed]
- Rahaman, M.; Day, D.; Bal, B.; Fu, Q.; Jung, S. Bioactive glass in tissue engineering. Acta Biomater. 2011, 7, 2355–2373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bi, L.; Jung, S.; Day, D.; Neidig, K.; Dusevich, V.; Eick, D.; Bonewald, L. Evaluation of bone regeneration, angiogenesis, and hydroxyapatite conversion in critical-sized rat calvarial defects implanted with bioactive glass scaffolds. J. Biomed. Mater. Res.—Part A 2012, 100A, 3267–3275. [Google Scholar] [CrossRef] [PubMed]
- Qazi, T.H.; Berkmann, J.C.; Schoon, J.; Geißler, S.; Duda, G.N.; Boccaccini, A.R.; Lippens, E. Dosage and composition of bioactive glasses differentially regulate angiogenic and osteogenic response of human MSCs. J. Biomed. Mater. Res.—Part A 2018, 106, 2827–2837. [Google Scholar] [CrossRef]
- Luthringer, B.J.C.; Willumeit-Römer, R. Effects of magnesium degradation products on mesenchymal stem cell fate and osteoblastogenesis. Gene 2016, 575, 9–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoppe, A.; Güldal, N.S.; Boccaccini, A.R.; Güldal, N.S.; Boccaccini, A.R. A review of the biological response to ionic dissolution products from bioactive glasses and glass-ceramics. Biomaterials 2011, 32, 2757–2774. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, S.; Vimalraj, S.; Vairamani, M.; Selvamurugan, N. Role of mesoporous wollastonite (calcium silicate) in mesenchymal stem cell proliferation and osteoblast differentiation: A cellular and molecular study. J. Biomed. Nanotechnol. 2015, 11, 1124–1138. [Google Scholar] [CrossRef] [PubMed]
- Mouthuy, P.A.; Snelling, S.J.B.; Dakin, S.G.; Milković, L.; Gašparović, A.Č.; Carr, A.J.; Žarković, N. Biocompatibility of implantable materials: An oxidative stress viewpoint. Biomaterials 2016, 109, 55–68. [Google Scholar] [CrossRef] [Green Version]
- Sheppard, A.J.; Barfield, A.M.; Barton, S.; Dong, Y. Understanding Reactive Oxygen Species in Bone Regeneration: A Glance at Potential Therapeutics and Bioengineering Applications. Front. Bioeng. Biotechnol. 2022, 10, 836764. [Google Scholar]
- Park, S.G.; Kim, J.H.; Xia, Y.; Sung, J.H. Generation of reactive oxygen species in adipose-derived stem cells: Friend or foe? Expert Opin. Ther. Targets 2011, 15, 1297–1306. [Google Scholar] [CrossRef]
- Atashi, F.; Modarressi, A.; Pepper, M.S. The role of reactive oxygen species in mesenchymal stem cell adipogenic and osteogenic differentiation: A review. Stem Cells Dev. 2015, 24, 1150–1163. [Google Scholar] [CrossRef]
















| Sample | [COOH/OH] | Temperature |
|---|---|---|
| PGSuc1 | 1:1 | 160 °C |
| PGSuc1b | 1:2.5 | 160 °C |
| PGSuc2 | 1:1 | 170 °C |
| PGSuc3 | 1:1 | 180 °C |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakiou, E.A.; Lazaridou, M.; Pouroutzidou, G.K.; Michopoulou, A.; Tsamesidis, I.; Liverani, L.; Arango-Ospina, M.; Beketova, A.; Boccaccini, A.R.; Kontonasaki, E.; et al. Poly(Glycerol Succinate) as Coating Material for 1393 Bioactive Glass Porous Scaffolds for Tissue Engineering Applications. Polymers 2022, 14, 5028. https://doi.org/10.3390/polym14225028
Nakiou EA, Lazaridou M, Pouroutzidou GK, Michopoulou A, Tsamesidis I, Liverani L, Arango-Ospina M, Beketova A, Boccaccini AR, Kontonasaki E, et al. Poly(Glycerol Succinate) as Coating Material for 1393 Bioactive Glass Porous Scaffolds for Tissue Engineering Applications. Polymers. 2022; 14(22):5028. https://doi.org/10.3390/polym14225028
Chicago/Turabian StyleNakiou, Eirini A., Maria Lazaridou, Georgia K. Pouroutzidou, Anna Michopoulou, Ioannis Tsamesidis, Liliana Liverani, Marcela Arango-Ospina, Anastasia Beketova, Aldo R. Boccaccini, Eleana Kontonasaki, and et al. 2022. "Poly(Glycerol Succinate) as Coating Material for 1393 Bioactive Glass Porous Scaffolds for Tissue Engineering Applications" Polymers 14, no. 22: 5028. https://doi.org/10.3390/polym14225028