Improving the Thermal Stability and Flame Retardancy of Epoxy Resins by Lamellar Cobalt Potassium Pyrophosphate
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis of Cobalt Potassium Pyrophosphate
2.3. Preparation of EP/LCPP Composites
2.4. Characterization
3. Results and Discussion
3.1. Structure and Morphology of Cobalt Potassium Pyrophosphate
3.2. Thermal Stability of EP/LCPP Composites
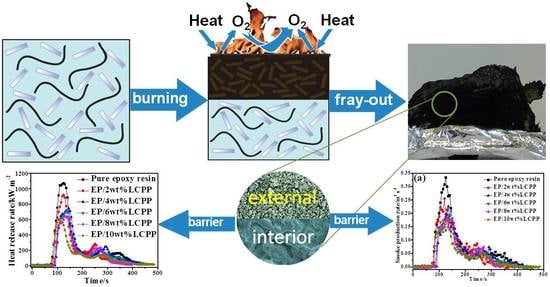
3.3. Combustion Performance of EP/LCPP Composites
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bekeshev, A.; Mostovoy, A.; Tastanova, L.; Kadykova, Y.; Kalganova, S.; Lopukhova, M. Reinforcement of epoxy composites with application of finely-ground ochre and electrophysical method of the composition modification. Polymers 2020, 12, 1437. [Google Scholar] [CrossRef] [PubMed]
- Umar, K.; Yaqoob, A.A.; Ibrahim, M.N.M.; Parveen, T.; Safian, M.T.-U. Environmental applications of smart polymer composites. In Smart Polymer Nanocomposites; Elsevier: Amsterdam, The Netherlands, 2021; pp. 295–312. [Google Scholar]
- Mostovoy, A.S.; Nurtazina, A.S.; Kadykova, Y.A.; Bekeshev, A.Z. Highly efficient plasticizers-antipirenes for epoxy polymers. Inorg. Mater. Appl. Res. 2019, 10, 1135–1139. [Google Scholar] [CrossRef]
- Kong, Q.H.; Wu, T.; Zhang, J.H.; Wang, D.Y. Simultaneously improving flame retardancy and dynamic mechanical properties of epoxy resin nanocomposites through layered copper phenylphosphate. Compos. Sci. Technol. 2018, 154, 136–144. [Google Scholar] [CrossRef]
- Bao, X.; Wu, F.; Wang, J. Thermal degradation behavior of epoxy resin containing modified carbon nanotubes. Polymers 2021, 13, 3332. [Google Scholar] [CrossRef]
- Peng, H.Y.; Wang, D.; Zhang, L.; Li, M.; Liu, M.; Wang, C.; Fu, S. Amorphous cobalt borate nanosheets grown on MoS2 nanosheet for simultaneously improving the flame retardancy and mechanical properties of polyacrylonitrile composite fiber. Compos. Part B Eng. 2020, 201, 108298. [Google Scholar] [CrossRef]
- Ding, J.M.; Zhang, Y.; Zhang, X.; Kong, Q.H.; Zhang, J.H.; Liu, H.; Zhang, F. Improving the flame-retardant efficiency of layered double hydroxide with disodium phenylphosphate for epoxy resin. J. Therm. Anal. Calorim. 2020, 140, 149–156. [Google Scholar] [CrossRef]
- Zhou, S.; Tao, R.; Dai, P.; Luo, Z.; He, M. Two-step fabrication of lignin-based flame retardant for enhancing the thermal and fire retardancy properties of epoxy resin composites. Polym. Composite. 2020, 41, 2025–2035. [Google Scholar] [CrossRef]
- Zhu, H.J.; Chen, Y.M.; Huang, S.; Wang, Y.; Yang, R.; Chai, H.; Zhu, F.; Kong, Q.H.; Zhang, Y.L.; Zhang, J.H. Suppressing fire hazard of poly(vinyl alcohol) based on (NH4)2[VO(HPO4)]2(C2O4)·5H2O with layered structure. J. Appl. Polym. Sci. 2021, 138, e51345. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, P.; Wang, F.; Kang, M.; Li, R.Q.; Mou, Y.R.; Huang, Y.H. Phase change materials based on polyethylene glycol supported by graphene-based mesoporous silica sheets. Appl. Therm. Eng. 2016, 101, 217–223. [Google Scholar] [CrossRef]
- Kong, Q.H.; Sun, Y.L.; Zhang, C.J.; Guan, H.M.; Zhang, J.H.; Wang, D.Y.; Zhang, F. Ultrathin iron phenyl phosphonate nanosheets with appropriate thermal stability for improving fire safety in epoxy. Compos. Sci. Technol. 2019, 182, 107748. [Google Scholar]
- Zhang, J.H.; Kong, Q.H.; Yang, L.W.; Wang, D.Y. Few layered Co(OH)2 ultrathin nanosheet-based polyurethane nanocomposites with reduced fire hazard: From eco-friendly flame retardance to sustainable recycling. Green Chem. 2016, 18, 3066–3074. [Google Scholar] [CrossRef]
- Xu, B.L.; Xu, W.Z.; Liu, Y.C.; Chen, R.; Li, W.; Wu, Y.; Yang, Z.T. Surface modification of -zirconium phosphate by zeolitic imidazolate frameworks-8 and its effect on improving the fire safety of polyurethane elastomer. Polym. Advan. Technol. 2018, 29, 2816–2826. [Google Scholar] [CrossRef]
- Kaul, P.K.; Samson, A.J.; Selvan, G.T.; Enoch, I.V.M.V.; Selvakumar, P.M. Synergistic effect of LDH in the presence of organophosphate on thermal and flammable properties of an epoxy nanocomposite. Appl. Clay Sci. 2017, 135, 234–243. [Google Scholar] [CrossRef]
- Kong, Q.H.; Zhu, H.J.; Fan, J.S.; Zheng, G.L.; Zhang, C.J.; Wang, Y.; Zhang, J.H. Boosting flame retardancy of epoxy resin composites through incorporating ultrathin nickel phenylphosphate nanosheets. J. Appl. Polym. Sci. 2021, 138, e50265. [Google Scholar] [CrossRef]
- Liu, X.Q.; Wang, D.Y.; Wang, X.L.; Chen, L.; Wang, Y.Z. Synthesis of functionalized alpha-zirconium phosphate modified with intumescent flame retardant and its application in poly(lactic acid). Polym. Degrad. Stabil. 2013, 98, 1731–1737. [Google Scholar] [CrossRef]
- Zhou, T.; Wu, T.; Xiang, H.N.; Li, Z.C.; Xu, Z.L.; Kong, Q.H.; Zhang, J.H.; Li, Z.; Pan, Y.T.; Wang, D.Y. Simultaneously improving flame retardancy and dynamic mechanical properties of epoxy resin nanocomposites through synergistic effect of zirconium phenylphosphate and POSS. J. Therm. Anal. Calorim. 2018, 35, 2117–2124. [Google Scholar]
- Nie, S.; Hu, Y.; Song, L.; He, Q.L.; Yang, D.D. Study on a novel and efficient flame retardant synergist-nanoporous nickel phosphates VSB-1 with intumescent flame retardants in polypropylene. Polym. Advan. Technol. 2008, 19, 489–495. [Google Scholar] [CrossRef]
- Ran, S.Y.; Guo, Z.H.; Chen, C.; Zhao, L.P.; Fang, Z.P. Carbon nanotube bridged cerium phenylphosphonate hybrids, fabrication and their effects on the thermal stability and flame retardancy of the HDPE/BFR composite. J. Mater. Chem. A 2014, 2, 2999–3007. [Google Scholar]
- Varhadi, P.; Kotwal, M.; Srinivas, D. Zirconium phenyl phosphonate phosphite as a highly active, reusable, solid acid catalyst for producing fatty acid polyol esters. Appl. Catal. A General. 2013, 462, 129–136. [Google Scholar] [CrossRef]
- Nie, S.B.; Song, L.; Bao, C.L.; Qian, X.D.; Guo, Y.Q.; Hong, N.N.; Hu, Y. Synergistic effects of ferric pyrophosphate (FePP) in intumescent flame-retardant polypropylene. Polym. Advan. Technol. 2011, 22, 870–876. [Google Scholar]
- Gao, M.Y.; Xue, Y.C.; Zhang, Y.T.; Zhu, C.X.; Yu, H.W.; Guo, X.M.; Sun, S.S.; Xiong, S.L.; Kong, Q.H.; Zhang, J.H. Growing Co-Ni-Se nanosheets on 3D carbon frameworks as advanced dualfunctional electrodes for supercapacitors and sodium ion batteries. Inorg. Chem. Front. 2022, 9, 3933–3942. [Google Scholar]
- Chen, J.L.; Guo, X.M.; Gao, M.Y.; Wang, J.; Sun, S.Q.; Xue, K.; Zhang, S.Y.; Liu, Y.J.; Zhang, J.H. Free-supporting dual-confined porous Si@c-ZIF@carbon nanofibersfor high-performance lithium-ion batteries. Chem. Commun. 2021, 57, 10580–10583. [Google Scholar]
- Kong, Q.H.; Wu, T.; Zhang, H.K.; Zhang, Y.; Zhang, M.M.; Si, T.Y.; Yang, L.; Zhang, J.H. Improving flame retardancy of IFR/PP composites through the synergistic effect of organic montmorillonite intercalation cobalt hydroxides modified by acidified chitosan. Appl. Clay Sci. 2017, 146, 230–237. [Google Scholar] [CrossRef]
- Bekeshev, A.; Mostovoy, A.; Kadykova, Y.; Akhmetova, M.; Tastanova, L.; Lopukhova, M. Development and analysis of the physicochemical and mechanical properties of diorite-reinforced epoxy composites. Polymers 2021, 13, 2421. [Google Scholar] [PubMed]
- Pan, Y.; Liu, C.; Wang, J.C.; Song, S.Q. Preparation of a novel synergistic flame retardant and its application in silicone rubber composites. Fire Mater. 2020, 44, 1135–1148. [Google Scholar]
- Qiu, S.L.; Hou, Y.B.; Xing, W.Y.; Ma, C.; Zhou, X.; Liu, L.X.; Kan, Y.C.; Yuen, R.K.; Hu, Y. Self-assembled supermolecular aggregate supported on boron nitride nanoplatelets for flame retardant and friction application. Chem. Eng. J. 2018, 349, 223–234. [Google Scholar]
- Zhou, K.Q.; Liu, C.K.; Gao, R. Polyaniline: A novel bridge to reduce the fire hazards of epoxy composites. Compos. Part A Appl. Sci. Manuf. 2018, 112, 432–443. [Google Scholar] [CrossRef]
- Wang, J.L.; Zhang, D.C.; Zhang, Y.; Cai, W.; Yao, C.X.; Hu, Y.; Hu, W.Z. Construction of multifunctional boron nitride nanosheet towards reducing toxic volatiles (CO and HCN) generation and fire hazard of thermoplastic polyurethane. J. Hazard Mater. 2019, 362, 482–494. [Google Scholar]
- Kong, Q.H.; Zhang, H.K.; Zheng, L.; Wang, D.Y.; Zhang, J.H. Effect on thermal and combustion behaviors of montmorillonite intercalation nickel compounds in polypropylene/IFR system. Polym. Adv. Technol. 2017, 28, 965–970. [Google Scholar] [CrossRef]
- Wan, J.T.; Gan, B.; Li, C.; Molina-Aldaregula, J.; Li, Z.; Wang, X.; Wang, D.Y. A novel biobased epoxy resin with high mechanical stiffness and low flammability: Synthesis, characterization and properties. J. Mater. Chem. A 2015, 3, 21907–21921. [Google Scholar]
- Kong, Q.H.; Zhu, H.J.; Huang, S.; Wu, T.; Zhu, F.; Zhang, Y.L.; Wang, Y.; Zhang, J.H. Influence of multiply modified FeCu-montmorillonite on fire safety and mechanical performances of epoxy resin nanocomposites. Thermochim. Acta 2022, 707, 179112. [Google Scholar] [CrossRef]
- Vababi, H.; Kandola, B.K.; Saeb, M.R. Flame retardancy index for thermoplastic composites. Polymers 2019, 11, 407. [Google Scholar] [CrossRef] [Green Version]
- Li, W.X.; Liao, D.J.; Hu, X.P.; Cheng, Z.; Xie, C.Q. Synergistic improvement of fire retardancy and mechanical properties of ferrocene-based polymer in intumescent polypropylene composite. Polym. Adv. Technol. 2019, 30, 2402–2413. [Google Scholar] [CrossRef]
- Zheng, L.; Wu, T.; Kong, Q.H.; Zhang, J.H.; Liu, H. Improving flame retardancy of PP/MH/RP composites through synergistic effect of organic CoAl-layered double hydroxide. J. Therm. Anal. Calorim. 2017, 129, 1039–1046. [Google Scholar] [CrossRef]
- Zhang, J.H.; Kong, Q.H.; Wang, D.Y. Simultaneously improving fire safety and mechanical properties of epoxy resin by Fe-CNTs via large-scale preparation. J. Mater. Chem. A 2018, 6, 6376–6386. [Google Scholar]
- Wang, L.Y.; Wei, Y.; Deng, H.B.; Lyu, R.Q.; Zhu, J.J.; Yang, Y.B. Synergistic flame retardant effect of barium phytate and intumescent flame retardant for epoxy resin. Polymers 2021, 13, 2900. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, H.K.; Sun, L.; Kong, Q.H.; Zhang, J.H. Flame-retardant effect of montmorillonite intercalation iron compounds in polypropylene/aluminum hydroxide composites system. J. Therm. Anal. Calorim. 2016, 124, 807–814. [Google Scholar] [CrossRef]







| Samples | Components | Flame Retardancy | |||
|---|---|---|---|---|---|
| EP/wt% | LCPP/wt% | LOI/vol% | UL-94 | ||
| (t1 + t2)/s | Rating | ||||
| Pure epoxy resin | 100 | 0 | 25.8 | >50.0 | NR |
| EP/1wt%LCPP | 99 | 1 | 27.2 | >50.0 | NR |
| EP/2wt%LCPP | 98 | 2 | 28.3 | >50.0 | NR |
| EP/4wt%LCPP | 96 | 4 | 29.8 | >50.0 | NR |
| EP/6wt%LCPP | 94 | 6 | 31.6 | 40.6 | V-1 |
| EP/8wt%LCPP | 92 | 8 | 33.8 | 32.3 | V-1 |
| EP/10wt%LCPP | 90 | 10 | 35.9 | 21.2 | V-1 |
| EP/12wt%LCPP | 88 | 12 | 34.2 | 28.4 | V-1 |
| Samples | T5%/°C | T50%/°C | Residues at 700 °C/wt% |
|---|---|---|---|
| Pure epoxy resin | 354 | 397 | 11.8 |
| EP/1wt%LCPP | 366 | 400 | 18.6 |
| EP/2wt%LCPP | 369 | 404 | 19.6 |
| EP/4wt%LCPP | 369 | 404 | 20.2 |
| EP/6wt%LCPP | 368 | 404 | 23.6 |
| EP/8wt%LCPP | 370 | 411 | 27.0 |
| EP/10wt%LCPP | 371 | 415 | 30.5 |
| EP/12wt%LCPP | 366 | 410 | 29.3 |
| Samples | TTI/ s | Peak HRR/ kW m−2 | THR/ MJ m−2 | PSPR/ m2 s−1 | TSP/ m2 kg−1 | FRI | Residues/wt% |
|---|---|---|---|---|---|---|---|
| pure epoxy resin | 77 | 1073.0 | 92.5 | 0.33 | 24.8 | 1.0 | 19.0 |
| EP/2wt%LCPP | 83 | 917.2 | 78.5 | 0.25 | 22.5 | 1.5 | 21.8 |
| EP/4wt%LCPP | 98 | 745.8 | 78.8 | 0.22 | 21.3 | 2.1 | 24.6 |
| EP/6wt%LCPP | 93 | 707.8 | 74.2 | 0.20 | 19.4 | 2.3 | 24.6 |
| EP/8wt%LCPP | 91 | 640.0 | 62.3 | 0.18 | 18.9 | 2.9 | 24.6 |
| EP/10wt%LCPP | 83 | 602.6 | 60.8 | 0.17 | 18.0 | 2.9 | 27.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, Q.; Li, L.; Zhang, M.; Chai, H.; Li, W.; Zhu, F.; Zhang, J. Improving the Thermal Stability and Flame Retardancy of Epoxy Resins by Lamellar Cobalt Potassium Pyrophosphate. Polymers 2022, 14, 4927. https://doi.org/10.3390/polym14224927
Kong Q, Li L, Zhang M, Chai H, Li W, Zhu F, Zhang J. Improving the Thermal Stability and Flame Retardancy of Epoxy Resins by Lamellar Cobalt Potassium Pyrophosphate. Polymers. 2022; 14(22):4927. https://doi.org/10.3390/polym14224927
Chicago/Turabian StyleKong, Qinghong, Lan Li, Manman Zhang, Huiyu Chai, Weixi Li, Fang Zhu, and Junhao Zhang. 2022. "Improving the Thermal Stability and Flame Retardancy of Epoxy Resins by Lamellar Cobalt Potassium Pyrophosphate" Polymers 14, no. 22: 4927. https://doi.org/10.3390/polym14224927