Dyeing Non-Recyclable Polyethylene Plastic with Photoacid Phycocyanobilin from Spirulina Algae: Ultrafast Photoluminescence Studies
Abstract
:1. Introduction
2. Result and Discussion
2.1. Photophysics of PCB in Neat Solvents
2.2. Interactions of PCB Dyes with LDPE Plastic Film: XPS Characterization
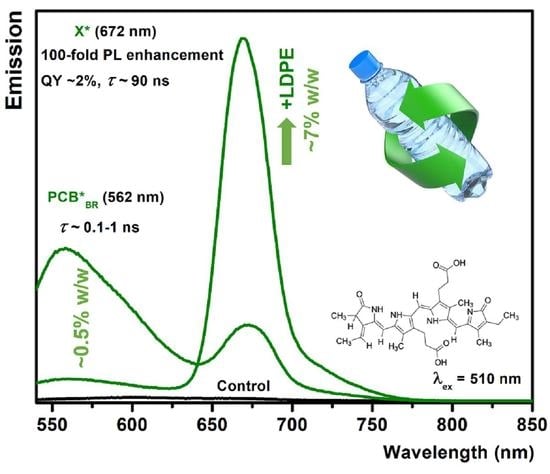
2.3. Loading and PL Characterization
2.4. PL and Time-Resolved PL Measurements
3. Conclusions
4. Method
4.1. Chemical and Reagent
4.2. Synthesis of PCB-Bound LDPE Films
4.3. DSC Measurement
4.4. XPS Measurement
4.5. Excited-State PL lifetime Measurements
4.6. Absolute PL Quantum Yield (QY) Measurements
4.7. DAS Measurements
4.8. Loading Characterization Methods
5. Supporting Information
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- White, W.; Sanborn, C.D.; Fabian, D.M.; Ardo, S. Conversion of Visible Light into Ionic Power Using Photoacid-Dye-Sensitized Bipolar Ion-Exchange Membranes. Joule 2018, 2, 94–109. [Google Scholar] [CrossRef]
- Sanborn, C.D.; Chacko, J.V.; Digman, M.; Ardo, S. Interfacial and Nanoconfinement Effects Decrease the Excited-State Acidity of Polymer-Bound Photoacids. Chem 2019, 5, 1648–1670. [Google Scholar] [CrossRef]
- White, W.; Sanborn, C.D.; Fabian, D.M.; Ardo, S. Observation of Photovoltaic Action from Photoacid-Modified Nafion Due to Light-Driven Ion Transport. J. Am. Chem. Soc. 2017, 139, 11726–11733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Shamsi, N.; Laptenok, S.P.; Bufaroosha, M.S.; Greish, Y.E.; Saleh, N. Time-Resolved Photoluminescence of 6-Thienyl-lumazine Fluorophores in Cellulose Acetate Nanofibers for Detection of Mercury Ions. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 222, 117–189. [Google Scholar] [CrossRef] [PubMed]
- Saleem, J.; Gae, P.; Barford, J.; Mckay, G. Development and Characterization of Novel Composite Membranes for Fuel Cell Applications. J. Mater. Chem. A 2013, 1, 14335–14343. [Google Scholar] [CrossRef]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, Use, and Fate of all Plastics ever Made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [Green Version]
- Miao, W.; Zhu, H.; Duan, T.; Chen, H.; Wu, F.; Jiang, L.; Wang, Z. High-Density Polyethylene Crystals with Double Melting Peaks Induced by Ultra-high-molecular-weight Polyethylene Fibre. R. Soc. Open Sci. 2018, 5, 180394. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, T.; Mishra, S. Studies on Extraction and Stability of C-Phycoerythrin from a Marine Cyanobacterium. Front. Sustain. Food Syst. 2020, 4, 102. [Google Scholar] [CrossRef]
- Gwizdala, M.; Krüger, T.P.; Wahadoszamen, M.; Gruber, J.M.; Van Grondelle, R. Phycocyanin: One complex, two states, two functions. J. Phys. Chem. Lett. 2018, 9, 1365–1371. [Google Scholar] [CrossRef] [Green Version]
- Theib, M.; Grupe, M.; Lamparter, T.; Mroginski, M.A.; Diller, R. Ultrafast proton release reaction and primary photochemistry of phycocyanobilin in solution observed with fs-time-resolved mid-IR and UV/Vis spectroscopy. Photochem. Photobiol. Sci. 2021, 20, 715–732. [Google Scholar]
- Hoffmann, E.A.; Fekete, Z.A.; Korugic-Karasz, L.S.; Karasz, F.E.; Wilusz, E. Theoretical and Experimental X-ray Photoelectron Spectroscopy Investigation of Ion-implanted Nafion. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 551–556. [Google Scholar] [CrossRef]
- Friedman, A.K.; Shi, W.; Losovyj, Y.; Siedle, A.R.; Baker, L.A. Mapping Microscale Chemical Heterogeneity in Nafion Membranes with X-ray Photoelectron Spectroscopy. J. Electrochem. Soc. 2018, 165, 733–741. [Google Scholar] [CrossRef]
- AlMaadeed, M.A.; Nógellová, Z.; Mičušík, M.; Novák, I.; Krupa, I. Mechanical, Sorption and Adhesive Properties of Composites based on Low Density Polyethylene Filled with Date Palm Wood Powder. Mater. Des. 2014, 53, 29–37. [Google Scholar] [CrossRef]
- Göller, A.H.; Strehlow, D.; Hermann, G. The Excited-State Chemistry of Phycocyanobilin: A Semiempirical Study. ChemPhysChem 2005, 6, 1259–1268. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, M.; Hermann, G.; Rentsch, S.; Strehlow, D.; Winter, S.; Chosrowjan, H. Excited-State Processes in Phycocyanobilin Studied by Femtosecond Spectroscopy. J. Phys. Chem. B 2000, 104, 1810–1816. [Google Scholar] [CrossRef]
- Mancini, J.A.; Kodali, G.; Jiang, J.; Reddy, K.R.; Lindsey, J.S.; Bryant, D.A.; Dutton, P.L.; Moser, C.C. Multi-step Excitation Energy Transfer Engineered in Genetic Fusions of Natural and Synthetic Light-Harvesting proteins. J. R. Soc. Interface 2016, 14, 20160896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dietzek, B.; Maksimenka, R.; Hermann, G.; Kiefer, W.; Popp, J.; Schmitt, M. The Excited-State Dynamics of Phycocyanobilin in Dependence on the Excitation Wavelength. ChemPhysChem 2004, 5, 1171–1177. [Google Scholar] [CrossRef] [PubMed]
- Singer, P.; Fey, S.; Göller, A.H.; Hermann, G.; Diller, R. Femtosecond Dynamics in the Lactim Tautomer of Phycocyanobilin: A Long-Wavelength Absorbing Model Compound for the Phytochrome Chromophore. ChemPhysChem 2014, 15, 3824–3831. [Google Scholar] [CrossRef]
- Radibratovic, M.; Minic, S.; Stanic-Vucinic, D.; Nikolic, M.; Milcic, M.; Velickovic, T.K. Stabilization of Human Serum Albumin by the Binding of Phycocyanobilin, a Bioactive Chromophore of Blue-Green Alga Spirulina: Molecular Dynamics and Experimental Study. PLoS ONE 2016, 11, e0167973. [Google Scholar] [CrossRef] [Green Version]
- Sanchez-Olivares, G.; Sanchez-Solis, A.; Manero, O.; Pérez-Chávez, R.; Jaramillo, M.; Alongi, J.; Carosio, F. Improving Mechanical Properties and Reaction to Fire of EVA/LLDPE Blends for Cable Applications with Melamine Triazine and Bentonite Clay. Materials 2019, 12, 2393. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.H.; Jung, J.S.; Kim, S.H. Dyeing and Antibacterial Properties of Chemically Recycled PET Thermal-Bonded Nonwovens Dyed with Terminalia chebula Dye. Polymers 2020, 12, 1675. [Google Scholar] [CrossRef] [PubMed]
- Sahare, S.; Veldurthi, N.; Singh, R.; Swarnkar, A.K.; Salunkhe, M.; Bhave, T. Enhancing the Efficiency of Flexible Dye-Sensitized Solar Cells utilizing Natural Dye Extracted from Azadirachta indica. Mater. Res. Express 2015, 2, 105903. [Google Scholar] [CrossRef]
- Watermann, T.; Elgabarty, H.; Sebastiani, D. Phycocyanobilin in solution—A solvent-triggered molecular switch. Phys. Chem. Chem. Phys. 2014, 16, 6146–6152. [Google Scholar] [CrossRef] [PubMed]




| Solvents | Soret-Band λe (nm) a | Q-Band λe (nm) a | PCBBR λf (nm) b | Q-Band λf (nm) b | Δν (cm−1) | τ (ns) c | η (cP) |
|---|---|---|---|---|---|---|---|
| Diethyl ether | 406 | 563 | 468 | 671 | 2859 | 1.82 | 0.24 |
| Acetonitrile | 406 | 560 | 502 | 670 | 2932 | 2.18 | 0.34 |
| Acetone | 406 | 561 | 501 | 671 | 2922 | 1.98 | 0.36 |
| Dichloromethane | 412 | 563 | 490 | 674 | 2925 | 2.12 | 0.41 |
| Ethyl acetate | 406 | 560 | 486 | 672 | 2977 | 1.74 | 0.45 |
| Methanol | 404 | 562 | 485 | 671 | 2890 | 2.44 | 0.54 |
| Dioxane | 409 | 560 | 470 | 674 | 3021 | 2.30 | 1.37 |
| Dimethyl sulfoxide | 410 | 560 | 485 | 674 | 3221 | 2.48 | 1.99 |
| Butanol | 402 | 562 | 530 | 673 | 2935 | 1.25 | 2.57 |
| Chlorobenzene | 416 | 565 | 470 | 675 | 1580 | 1.63 | 7.68 |
| Mineral oil | 411 | 563 | 465 | 674 | 1503 | 1.30 | 84 |
| Glycerol | 350 | - | 462 | - | 6926 | 1.04 | 954 |
| Samples | λobs (nm) | τ1 (ns) | f1% | τ2 (ns) | f2% | τ3 (ns) | f3% | τaverage (ns) | Chi-Square |
|---|---|---|---|---|---|---|---|---|---|
| PCB in MeOH (10 µM) | 560 | 0.1 | 40 | 1.9 | 22 | 5.2 | 38 | 2.4 | 1.034 |
| 672 | 0.1 | 42 | 1.8 | 13 | 5.2 | 45 | 2.6 | 0.985 | |
| PCB@LDPE (10 µM) | 560 | 0.6 | 55 | 2.8 | 35 | 8.7 | 10 | 2.2 | 1.404 |
| 672 | 0.3 | 64 | 1.5 | 16 | 6.1 | 20 | 1.7 | 1.148 | |
| PCB@LDPE (100 µM) | 560 | 0.8 | 75 | 1.7 | 24 | 15.6 | 1 | 1.2 | 1.090 |
| 672 | 1.1 | 26 | 6.6 | 71 | 16.6 | 3 | 5.5 | 1.240 | |
| PCB@LDPE (1000 µM) | 560 | 0.4 | 30 | 1.2 | 60 | 4.7 | 10 | 1.3 | 1.333 |
| 672 | 1.1 | 2 | 5.2 | 38 | 7.8 | 60 | 6.7 | 1.168 |
| % Loaded PCB/per LDPE (w/w) | τ/ns Excited-State Lifetimes | DAS Maximum (nm) | DAS Amplitudes | %X* | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PCBBR* 1 | PCBBR* 2 | X* | PCBBR* 1 | PCBBR* 2 | X* | PCBBR* 1 | PCBBR* 2 | X* | ||
| 0.08% | 0.1 | 1.1 | 4.9 | 550 | 560 | 675 | 0.20 | 0.50 | 0.30 | 30% |
| 0.44% | 0.1 | 1.0 | 6.6 | 550 | 560 | 675 | 0.05 | 0.65 | 0.30 | 30% |
| 6.92% | 0.5 | 3.8 | 90 | 550 | 560 | 675 | 0.05 | 0.05 | 0.90 | 90% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhefeiti, M.; Chandra, F.; Gupta, R.K.; Saleh, N. Dyeing Non-Recyclable Polyethylene Plastic with Photoacid Phycocyanobilin from Spirulina Algae: Ultrafast Photoluminescence Studies. Polymers 2022, 14, 4811. https://doi.org/10.3390/polym14224811
Alhefeiti M, Chandra F, Gupta RK, Saleh N. Dyeing Non-Recyclable Polyethylene Plastic with Photoacid Phycocyanobilin from Spirulina Algae: Ultrafast Photoluminescence Studies. Polymers. 2022; 14(22):4811. https://doi.org/10.3390/polym14224811
Chicago/Turabian StyleAlhefeiti, Maryam, Falguni Chandra, Ravindra Kumar Gupta, and Na’il Saleh. 2022. "Dyeing Non-Recyclable Polyethylene Plastic with Photoacid Phycocyanobilin from Spirulina Algae: Ultrafast Photoluminescence Studies" Polymers 14, no. 22: 4811. https://doi.org/10.3390/polym14224811