Assembling of Metal-Polymer Nanocomposites in Irradiated Solutions of 1-Vinyl-1,2,4-triazole and Au(III) Ions: Features of Polymerization and Nanoparticles Formation
Abstract
:1. Introduction
2. Materials and Methods
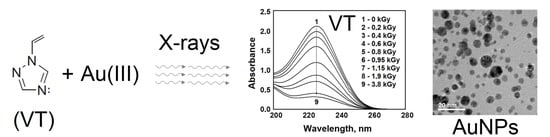
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xia, Y.; Xiong, Y.; Lim, B.; Skrabalak, S.E. Shape-Controlled Synthesis of Metal Nanocrystals: Simple Chemistry Meets Complex Physics? Angew. Chem. Int. Ed. 2008, 48, 60–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elahi, N.; Kamali, M.; Baghersad, M.H. Recent biomedical applications of gold nanoparticles: A review. Talanta 2018, 184, 537–556. [Google Scholar] [CrossRef] [PubMed]
- Amendola, V.; Pilot, R.; Frasconi, M.; Maragò, O.M.; Iatì, M.A. Surface plasmon resonance in gold nanoparticles: A review. J. Phys. Condens. Matter 2017, 29, 203002. [Google Scholar] [CrossRef] [PubMed]
- Ishida, T.; Murayama, T.; Taketoshi, A.; Haruta, M. Importance of Size and Contact Structure of Gold Nanoparticles for the Genesis of Unique Catalytic Processes. Chem. Rev. 2020, 120, 464–525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehravani, B.; Ribeiro, A.I.; Zille, A. Gold Nanoparticles Synthesis and Antimicrobial Effect on Fibrous Materials. Nanomaterials 2021, 11, 1067. [Google Scholar] [CrossRef]
- Ielo, I.; Rando, G.; Giacobello, F.; Sfameni, S.; Castellano, A.; Galletta, M.; Drommi, D.; Rosace, G.; Plutino, M.R. Synthesis, Chemical–Physical Characterization, and Biomedical Applications of Functional Gold Nanoparticles: A Review. Molecules 2021, 26, 5823. [Google Scholar] [CrossRef] [PubMed]
- Sibuyi, N.R.S.; Moabelo, K.L.; Fadaka, A.O.; Meyer, S.; Onani, M.O.; Madiehe, A.M.; Meyer, M. Multifunctional Gold Nanoparticles for Improved Diagnostic and Therapeutic Applications: A Review. Nanoscale Res. Lett. 2021, 16, 174. [Google Scholar] [CrossRef]
- Buonerba, A.; Grassi, A. Trends in Sustainable Synthesis of Organics by Gold Nanoparticles Embedded in Polymer Matrices. Catalysts 2021, 11, 714. [Google Scholar] [CrossRef]
- Zezin, A.A. Synthesis of hybrid materials in polyelectrolyte matrixes: Control over sizes and spatial organization of metallic nanostructures. Polym. Sci. Ser. C 2016, 58, 118–130. [Google Scholar] [CrossRef]
- Das, S.S.; Bharadwaj, P.; Bilal, M.; Barani, M.; Rahdar, A.; Taboada, P.; Bungau, S.; Kyzas, G.Z. Stimuli-Responsive Polymeric Nanocarriers for Drug Delivery, Imaging, and Theragnosis. Polymers 2020, 12, 1397. [Google Scholar] [CrossRef]
- Ansari, S.A.; Parveen, N.; Han, T.H.; Ansari, M.O.; Cho, M.H. Fibrous polyaniline@manganese oxide nanocomposites as supercapacitor electrode materials and cathode catalysts for improved power production in microbial fuel cells. Phys. Chem. Chem. Phys. 2016, 18, 9053–9060. [Google Scholar] [CrossRef] [PubMed]
- Parveen, N.; Ansari, M.O.; Cho, M.H. Route to High Surface Area, Mesoporosity of Polyaniline–Titanium Dioxide Nanocomposites via One Pot Synthesis for Energy Storage Applications. Ind. Eng. Chem. Res. 2016, 55, 116–124. [Google Scholar] [CrossRef]
- Karoutsos, V.; Koutselas, I.; Orfanou, P.; Mpatzaka, T.; Vasileiadis, M.; Vassilakopoulou, A.; Vainos, N.A.; Perrone, A. One-pot synthesis and transfer of PMMA/Ag photonic nanocomposites by pulsed laser deposition. Appl. Phys. A 2015, 120, 707–716. [Google Scholar] [CrossRef]
- Chmielewski, A.G.; Chmielewska, D.K.; Michalik, J.; Sampa, M.H. Prospects and challenges in application of gamma, electron and ion beams in processing of nanomaterials. Nucl. Instrum. Methods Phys. Res. B 2007, 265, 339–346. [Google Scholar] [CrossRef]
- Zezin, A.A.; Emel’yanov, A.I.; Prozorova, G.F.; Zezina, E.A.; Feldman, V.I.; Abramchuk, S.S.; Pozdnyakov, A.S. A one-pot radiation-chemical synthesis of metal-polymeric nanohybrides in solutions of vinyltriazole containing gold ions. Mendeleev Commun. 2019, 29, 158–159. [Google Scholar] [CrossRef]
- Zezin, A.A.; Zharikov, A.A.; Emel’yanov, A.I.; Pozdnyakov, A.S.; Prozorova, G.F.; Abramchuk, S.S.; Zezina, E.A. One-Pot Preparation of Metal–Polymer Nanocomposites in Irradiated Aqueous Solutions of 1-Vinyl-1,2,4-triazole and Silver Ions. Polymers 2021, 13, 4235. [Google Scholar] [CrossRef]
- Ashfaq, A.; Clochard, M.-C.; Coqueret, X.; Dispenza, C.; Driscoll, M.S.; Ulański, P.; Al-Sheikhly, M. Polymerization Reactions and Modifications of Polymers by Ionizing Radiation. Polymers 2020, 12, 2877. [Google Scholar] [CrossRef]
- Rosiak, J.M.; Ulański, P. Synthesis of hydrogels by irradiation of polymers in aqueous solution. Radiat. Phys. Chem. 1999, 55, 139–151. [Google Scholar] [CrossRef]
- Coqueret, X. Radiation-induced polymerization. In Applications of Ionizing Radiation in Materials Processing, 1st ed.; Sun, Y., Ed.; Institute of Nuclear Chemistry and Technology: Warszawa, Poland, 2017; pp. 143–165. ISBN 978-83-933935-9-6. [Google Scholar]
- Belloni, J. Nucleation, growth and properties of nanoclusters studied by radiation chemistry. Catal. Today 2006, 113, 141–156. [Google Scholar] [CrossRef]
- Freitas de Freitas, L.; Varca, G.H.C.; Dos Santos Batista, J.G.; Benévolo Lugão, A. An Overview of the Synthesis of Gold Nanoparticles Using Radiation Technologies. Nanomaterials 2018, 8, 939. [Google Scholar] [CrossRef]
- Toro-Gonalez, M.; Clifford, D.M.; Molina, M.C.; Castano, C.E.; Rojas, J.V. New concept of radiolytic synthesis of gold nanoparticles in continuous flow. Rad. Phys. Chem. 2021, 188, 109614. [Google Scholar] [CrossRef]
- Bondaz, L.; Fontaine, P.; Muller, F.; Pantoustier, N.; Perrin, P.; Morfin, I.; Goldmann, M.; Cousin, F. Controlled synthesis of gold nanoparticles in copolymers nanomolds by X-ray radiolysis. Langmuir 2020, 36, 6132–6144. [Google Scholar] [CrossRef]
- Prozorova, G.F.; Pozdnyakov, A.S. Synthesis, Properties, and Biological Activity of Poly(1-vinyl-1,2,4-triazole) and Silver Nanocomposites Based on It. Polym. Sci. Ser. C 2022, 64, 62–72. [Google Scholar] [CrossRef]
- Pozdnyakov, A.S.; Ivanova, A.A.; Emel’yanov, A.I.; Bolgova, Y.I.; Trofimova, O.M.; Prozorova, G.F. Water-soluble stable polymer nanocomposites with AuNPs based on the functional poly(1-vinyl-1,2,4-triazole-co-N-vinylpyrrolidone). J. Organomet. Chem. 2020, 922, 121352. [Google Scholar] [CrossRef]
- Zezin, A.; Danelyan, G.; Emel’yanov, A.; Zharikov, A.; Prozorova, G.; Zezina, E.; Korzhova, S.; Fadeeva, T.; Abramchuk, S.; Shmakova, N.; et al. Synthesis of antibacterial polymer metal hybrids in irradiated poly-1-vinyl-1,2,4-triazole complexes with silver ions: pH tuning of nanoparticle sizes. Appl. Organomet. Chem. 2022, 36, e6581. [Google Scholar] [CrossRef]
- Zharikov, A.A.; Vinogradov, R.A.; Zezina, E.A.; Pozdnyakov, A.S.; Feldman, V.I.; Vasiliev, A.L.; Zezin, A.A. The radiation-induced preparation of ultrasmall gold nanoparticles in Au(III) complexes with units of poly(1-vinyl-1,2,4-triazole) and poly(1-vinyl-1,2,4-triazole)–poly(acrylic acid). Colloids Interface Sci. Commun. 2022, 47, 100602. [Google Scholar] [CrossRef]
- Tables of X-ray Mass Attenuation Coefficients and Mass Energy-Absorption Coefficients from 1 keV to 20 MeV for Elements Z = 1 to 92 and 48 Additional Substances of Dosimetric Interest. (X-Ray Mass Attenuation Coefficients). Available online: http://www.nist.gov/pml/data/xraycoef (accessed on 17 February 2022).
- Usher, A.; McPhail, D.C.; Brugger, J.A. spectrophotometric study of aqueous Au(III) halide–hydroxide complexes at 25–80 °C. Geochim. Cosmochim. Acta 2009, 73, 3359–3380. [Google Scholar] [CrossRef]
- Ershov, B.G.; Abkhalimov, E.A. Mechanism of silver nucleation upon the radiation-induced reduction of its ions in polyphosphate-containing aqueous solutions. Colloid J. 2006, 68, 417–424. [Google Scholar] [CrossRef]
- Hanawalt, J.D.; Rinn, H.W.; Frevel, L.K. Chemical Analysis by X-Ray Diffraction. Ind. Eng. Chem. Anal. Ed. 1938, 10, 457–512. [Google Scholar] [CrossRef]
- Feldman, V.I.; Zezin, A.A.; Abramchuk, S.S.; Zezina, E.A. X-ray Induced Formation of Metal Nanoparticles from Interpolyelectrolyte Complexes with Copper and Silver Ions: The Radiation-Chemical Contrast. Phys. Chem. C 2013, 117, 7286–7293. [Google Scholar] [CrossRef]
- Wardman, P. Reduction Potentials of One-Electron Couples Involving Free Radicals in Aqueous Solution. J. Phys. Chem. Ref. Data 1989, 18, 1637–1755. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Richards, V.N.; Shields, S.P.; Buhro, W.E. Kinetics and Mechanisms of Aggregative Nanocrystal Growth. Chem. Mater. 2014, 26, 5–21. [Google Scholar] [CrossRef]
- Gachard, E.; Remita, H.; Khatouri, J.; Keita, B.; Nadjo, L.; Belloni, J. Radiation-induced and chemical formation of gold clusters. New J. Chem. 1998, 22, 1257–1265. [Google Scholar] [CrossRef]
- Dey, G.R.; El Omar, A.K.; Jacob, J.A.; Mostafavi, M.; Belloni, J. Mechanism of Trivalent Gold Reduction and Reactivity of Transient Divalent and Monovalent Gold Ions Studied by Gamma and Pulse Radiolysis. J. Phys. Chem. A 2011, 115, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Mosseri, S.; Henglein, A.; Janata, E. Reduction of dicyanoaurate(I) in aqueous solution: Formation of nonmetallic clusters and colloidal gold. J. Phys. Chem. 1989, 93, 6791–6795. [Google Scholar] [CrossRef]
- CRC Handbook of Chemistry and Physics, 90th ed.; CRC Press: Boca Raton, FL, USA, 2010; p. 2758.
- Remita, S.; Archirel, P.; Mostafavi, M. Evaluation of the redox potential of Ag1I(CN)2-/Ag10(CN)22- in aqueous solution. J. Phys. Chem. 1995, 99, 13198–13202. [Google Scholar] [CrossRef]
- Texier, I.; Remita, S.; Archirel, P.; Mostafavi, M. Reduction of AgI1(NH3)2+ to Ag01(NH3)2 in solution. Redox potential and spectral study. J. Phys. Chem. 1996, 100, 12472–12476. [Google Scholar] [CrossRef]
- Henglein, A. The reactivity of silver atoms in aqueous solutions. Ber. Bunsenges. Phys. Chem. 1977, 81, 556–561. [Google Scholar] [CrossRef]
- Fischer, H.; Radom, L. Factors Controlling the Addition of Carbon-Centered Radicals to Alkenes–An Experimental and Theoretical Perspective. Angew. Chem. Int. Ed. 2001, 40, 1340–1371. [Google Scholar] [CrossRef]
- Stetsyshyn, Y.; Awsiuk, K.; Kusnezh, V.; Raczkowska, J.; Jany, B.R.; Kostruba, A.; Harhay, K.; Ohar, H.; Lishchynskyi, O.; Shymborska, Y.; et al. Shape-Controlled synthesis of silver nanoparticles in temperature-responsive grafted polymer brushes for optical applications. Appl. Surf. Sci. 2019, 463, 1124–1133. [Google Scholar] [CrossRef]
- Kochkar, H.; Aouine, M.; Ghorbel, A.; Berhault, G. Shape-Controlled Synthesis of Silver and Palladium Nanoparticles Using β-Cyclodextrin. J. Phys. Chem. C. 2011, 115, 11364–11373. [Google Scholar] [CrossRef]
- Mayer, A.B.R. Colloidal Metal Nanoparticles Dispersed in Amphiphilic Polymers. Polym. Adv. Technol. 2001, 12, 96–106. [Google Scholar] [CrossRef]










| Ratio | 1/40 | 1/80 | 1/160 | 1/320 |
|---|---|---|---|---|
| C(Au(III)), mol/L | 2.6 × 10−3 | 1.3 × 10−3 | 6.6 × 10−4 | 3.3 × 10−4 |
| C(VT), mol/L | 1.05 × 10−1 | 1.05 × 10−1 | 1.05 × 10−1 | 1.05 × 10−1 |
| [VT]/[Au(III)] Ratio | 1/40 | 1/80 | 1/160 | 1/320 | without Au(III) |
|---|---|---|---|---|---|
| G(-VT) molecules/100 eV | 70 (4) | 80 (6) | 320 (3) | 2.0 × 10−3 (3) | 2.4 × 10−3 (4) [16] |
| Inhibition period, kGy | |||||
| (polymerization) | ~1.8 | ~1.3 | <0.4 | - | - |
| Induction period | |||||
| (formation of AuNPs) | ~2.8 | ~1.6 | ~0.6 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zharikov, A.A.; Zezina, E.A.; Vinogradov, R.A.; Pozdnyakov, A.S.; Feldman, V.I.; Chvalun, S.N.; Vasiliev, A.L.; Zezin, A.A. Assembling of Metal-Polymer Nanocomposites in Irradiated Solutions of 1-Vinyl-1,2,4-triazole and Au(III) Ions: Features of Polymerization and Nanoparticles Formation. Polymers 2022, 14, 4601. https://doi.org/10.3390/polym14214601
Zharikov AA, Zezina EA, Vinogradov RA, Pozdnyakov AS, Feldman VI, Chvalun SN, Vasiliev AL, Zezin AA. Assembling of Metal-Polymer Nanocomposites in Irradiated Solutions of 1-Vinyl-1,2,4-triazole and Au(III) Ions: Features of Polymerization and Nanoparticles Formation. Polymers. 2022; 14(21):4601. https://doi.org/10.3390/polym14214601
Chicago/Turabian StyleZharikov, Alexey A., Elena A. Zezina, Rodion A. Vinogradov, Alexander S. Pozdnyakov, Vladimir I. Feldman, Sergey N. Chvalun, Alexander L. Vasiliev, and Alexey A. Zezin. 2022. "Assembling of Metal-Polymer Nanocomposites in Irradiated Solutions of 1-Vinyl-1,2,4-triazole and Au(III) Ions: Features of Polymerization and Nanoparticles Formation" Polymers 14, no. 21: 4601. https://doi.org/10.3390/polym14214601