Facile One-Step Synthesis of PVDF Bead-on-String Fibers by Pressurized Gyration for Reusable Face Masks
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Characterization of PVDF SOLUTIONS
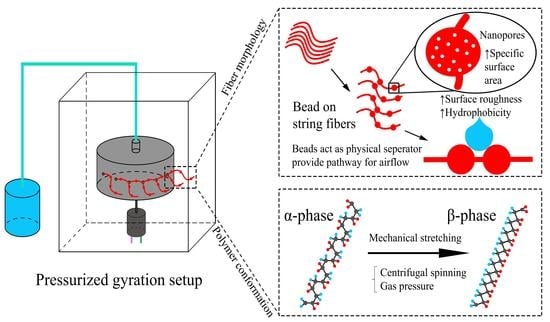
2.3. Pressurized Gyration

2.4. Characterization
2.4.1. Scanning Electron Microscopy (SEM)
2.4.2. Attenuated Total Reflection Fourier Transform Infrared Spectroscopy (ATR-FTIR)
2.4.3. Static Surface Hydrophobicity Measurement
3. Results and Discussion
3.1. Influence of the PG Solution Parameters on Fiber Morphology
3.1.1. Effects of Polymer Concentrations on Fiber Morphology
3.1.2. Effects of Solvent Ratio on Fiber Morphology
3.2. β-Phase Formation of PG PVDF
3.3. Water Repellency
3.4. Yields and Production Rate
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Z.; Ji, D.; He, H.; Ramakrishna, S. Electrospun Ultrafine Fibers for Advanced Face Masks. Mater. Sci. Eng. R Rep. 2021, 143, 100594. [Google Scholar] [CrossRef] [PubMed]
- Seidi, F.; Deng, C.; Zhong, Y.; Liu, Y.; Huang, Y.; Li, C.; Xiao, H. Functionalized Masks: Powerful Materials against COVID-19 and Future Pandemics. Small 2021, 17, 2102453. [Google Scholar] [CrossRef] [PubMed]
- Allison, A.L.; Ambrose-Dempster, E.; Bawn, M.; Arredondo, M.C.; Chau, C.; Chandler, K.; Dobrijevic, D.; Aparasi, T.D.; Hailes, H.C.; Lettieri, P.; et al. The Impact and Effectiveness of the General Public Wearing Masks to Reduce the Spread of Pandemics in the UK: A Multidisciplinary Comparison of Single-Use Masks versus Reusable Face Masks. UCL Open Environ. 2021, 3, 1. [Google Scholar] [CrossRef]
- Woon, W.; Leung, F.; Sun, Q. Electrostatic Charged Nanofiber Filter for Filtering Airborne Novel Coronavirus (COVID-19) and Nano-Aerosols. Sep. Purif. Technol. 2020, 250, 116886. [Google Scholar]
- Wang, Z.; Zhao, C.; Pan, Z. Porous Bead-on-String Poly(Lactic Acid) Fibrous Membranes for Air Filtration. J. Colloid Interface Sci. 2015, 441, 121–129. [Google Scholar] [CrossRef]
- Gao, X.; Wen, S.; Yang, B.; Xue, J.; Wang, H. Enhanced Air Filtration Performance under High-Humidity Condition through Electrospun Membranes with Optimized Structure. Chin. J. Chem. Eng. 2020, 28, 1788–1795. [Google Scholar] [CrossRef]
- Han, W.; Rao, D.; Gao, H.; Yang, X.; Fan, H.; Li, C.; Dong, L.; Meng, H. Green-Solvent-Processable Biodegradable Poly(Lactic Acid) Nanofibrous Membranes with Bead-on-String Structure for Effective Air Filtration: “Kill Two Birds with One Stone”. Nano Energy 2022, 97, 107237. [Google Scholar] [CrossRef]
- Zulfi, A.; Hapidin, D.A.; Munir, M.M.; Iskandar, F.; Khairurrijal, K. The Synthesis of Nanofiber Membranes from Acrylonitrile Butadiene Styrene (ABS) Waste Using Electrospinning for Use as Air Filtration Media. RSC Adv. 2019, 9, 30741–30751. [Google Scholar] [CrossRef] [Green Version]
- Mahalingam, S.; Edirisinghe, M. Forming of Polymer Nanofibers by a Pressurised Gyration Process. Macromol. Rapid Commun. 2013, 34, 1134–1139. [Google Scholar] [CrossRef] [Green Version]
- Heseltine, P.L.; Ahmed, J.; Edirisinghe, M. Developments in Pressurized Gyration for the Mass Production of Polymeric Fibers. Macromol. Mater. Eng. 2018, 303, 1800218. [Google Scholar] [CrossRef] [Green Version]
- Raimi-Abraham, B.T.; Mahalingam, S.; Edirisinghe, M.; Craig, D.Q. Generation of poly(N-vinylpyrrolidone) nanofibres using pressurised gyration. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 1, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Illangakoon, U.E.; Mahalingam, S.; Matharu, R.K.; Edirisinghe, M. Evolution of Surface Nanopores in Pressurised Gyrospun Polymeric Microfibers. Polymers 2017, 9, 508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, X.; Edirisinghe, M.; Mahalingam, S. Beads, Beaded-Fibres and Fibres: Tailoring the Morphology of Poly(Caprolactone) Using Pressurised Gyration. Mater. Sci. Eng. C 2016, 69, 1373–1382. [Google Scholar] [CrossRef]
- Wypych, G. (Ed.) PVDF poly(vinylidene fluoride). In Handbook of Polymers; Elsevier: Oxford, UK, 2012; pp. 604–608. [Google Scholar]
- Kang, G.D.; Cao, Y.M. Application and Modification of Poly(Vinylidene Fluoride) (PVDF) Membranes—A Review. J. Membr. Sci. 2014, 463, 145–165. [Google Scholar] [CrossRef]
- Sanyal, A.; Sinha-Ray, S. Ultrafine PVDF Nanofibers for Filtration of Air-Borne Particulate Matters: A Comprehensive Review. Polymers 2021, 13, 1864. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Park, H.; Lee, J.H. Recent Structure Development of Poly(Vinylidene Fluoride)-Based Piezoelectric Nanogenerator for Self-Powered Sensor. Actuators 2020, 9, 57. [Google Scholar] [CrossRef]
- Wang, W.; Pang, J.; Su, J.; Li, F.; Li, Q.; Wang, X.; Wang, J.; Ibarlucea, B.; Liu, X.; Li, Y.; et al. Applications of Nanogenerators for Biomedical Engineering and Healthcare Systems. InfoMat 2022, 4, e12262. [Google Scholar] [CrossRef]
- Liu, G.; Nie, J.; Han, C.; Jiang, T.; Yang, Z.; Pang, Y.; Xu, L.; Guo, T.; Bu, T.; Zhang, C.; et al. Self-Powered Electrostatic Adsorption Face Mask Based on a Triboelectric Nanogenerator. ACS Appl. Mater. Interfaces 2018, 10, 7126–7133. [Google Scholar] [CrossRef]
- Ibtehaj, K.; Hj Jumali, M.H.; Al-Bati, S. A Novel Facile Preparation Method of Self-Polarized Poly(Vinylidene Fluorides) Nanofiber for High-Performance Piezoelectric Nanogenerator. Polymer 2020, 208, 122956. [Google Scholar] [CrossRef]
- He, Z.; Rault, F.; Lewandowski, M.; Mohsenzadeh, E.; Salaün, F. Electrospun PVDF Nanofibers for Piezoelectric Applications: A Review of the Influence of Electrospinning Parameters on the β Phase and Crystallinity Enhancement. Polymers 2021, 13, 174. [Google Scholar] [CrossRef]
- Ibtehaj, K.; Jumali, M.H.H.; Al-Bati, S.; Ooi, P.C.; Al-Asbahi, B.A.; Ahmed, A.A.A. Effect of β-Chain Alignment Degree on the Performance of Piezoelectric Nanogenerator Based on Poly(Vinylidene Fluoride) Nanofiber. Macromol. Res. 2022, 30, 172–182. [Google Scholar] [CrossRef]
- Su, Q.; Jiang, Z.; Li, B. A Mixed Solvent Approach to Make Poly(Vinylidene Fluoride) Nanofibers with High β-Phase Using Solution Blow Spinning. High Perform. Polym. 2020, 32, 1160–1168. [Google Scholar] [CrossRef]
- Martins, P.; Lopes, A.C.; Lanceros-Mendez, S. Electroactive Phases of Poly(vinylidene fluoride): Determination, Processing and Applications. Prog. Polym. Sci. 2014, 39, 683–706. [Google Scholar] [CrossRef]
- Mahalingam, S.; Wang, K.; Cheong, Y.K.; Canales, E.; Ren, G.G.; Cloutman-Green, E.; Edirisinghe, M.; Ciric, L. Gyrospun Antimicrobial Nanoparticle Loaded Fibrous Polymeric Filters. Mater. Sci. Eng. C 2017, 74, 315–324. [Google Scholar]
- Kumar, C.; Viswanath, P. Solvent Driven Polymorphism in Langmuir and Langmuir Schaefer Film of Poly(vinylidene fluoride). Eur. Polym. J. 2017, 86, 132–142. [Google Scholar] [CrossRef]
- Dai, Y.; Ahmed, J.; Delbusso, A.; Edirisinghe, M. Nozzle-Pressurized Gyration: A Novel Fiber Manufacturing Process. Macromol. Mater. Eng. 2022, 178, 107846. [Google Scholar]
- Mellado, P.; McIlwee, H.A.; Badrossamay, M.R.; Goss, J.A.; Mahadevan, L.; Kit Parker, K. A Simple Model for Nanofiber Formation by Rotary Jet-spinning. Appl. Phys. Lett. 2011, 99, 203107. [Google Scholar] [CrossRef] [Green Version]
- Matharu, R.K.; Charani, Z.; Ciric, L.; Illangakoon, U.E.; Edirisinghe, M. Antimicrobial activity of tellurium-loaded polymeric fiber meshes. J. Appl. Polym. Sci. 2018, 135, 4636831. [Google Scholar] [CrossRef] [Green Version]
- Badrossamay, M.R.; McIlwee, H.A.; Goss, J.A.; Parker, K.K. Nanofiber Assembly by Rotary Jet-Spinning. Nano Lett. 2010, 10, 2257–2261. [Google Scholar] [CrossRef] [Green Version]
- Dorrer, C.; Rühe, J. Some Thoughts on Superhydrophobic Wetting. Soft Matter 2009, 5, 51–61. [Google Scholar] [CrossRef]
- Deng, Y.; Mager, D.; Bai, Y.; Zhou, T.; Liu, Z.; Wen, L.; Wu, Y.; Korvink, J.G. Inversely Designed Micro-Textures for Robust Cassie–Baxter Mode of Super-Hydrophobicity. Comput. Methods Appl. Mech. Eng. 2018, 341, 113–132. [Google Scholar] [CrossRef]
- Peng, H. Synthesis and Application of Fluorine-containing Polymers With Low Surface Energy. Polym. Rev. 2019, 59, 739–757. [Google Scholar] [CrossRef]









| Sample Name | Polymer Concentrations [% (w/v)] | Acetone to DMF Ratio [v/v] | Viscosity [mPa s] | Shear rate [s−1] |
|---|---|---|---|---|
| 15P5A5D | 15 | 5/5 | 17.80 ± 0.21 | 34 |
| 15P6A4D | 15 | 6/4 | 19.74 ± 0.25 | 34 |
| 15P7A3D | 15 | 7/3 | 17.33 ± 0.20 | 34 |
| 20P5A5D | 20 | 5/5 | 30.70 ± 0.67 | 12 |
| 20P6A4D | 20 | 6/4 | 55.29 ± 3.80 | 12 |
| 20P7A3D | 20 | 7/3 | 45.43 ± 3.56 | 12 |
| 25P5A5D | 25 | 5/5 | 100.51 ± 1.95 | 6 |
| 25P6A4D | 25 | 6/4 | 140.80 ± 17.01 | 5 |
| 25P7A3D | 25 | 7/3 | 102.00 ± 1.39 | 6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, R.; Dai, Y.; Ahmed, J.; Edirisinghe, M. Facile One-Step Synthesis of PVDF Bead-on-String Fibers by Pressurized Gyration for Reusable Face Masks. Polymers 2022, 14, 4498. https://doi.org/10.3390/polym14214498
Huang R, Dai Y, Ahmed J, Edirisinghe M. Facile One-Step Synthesis of PVDF Bead-on-String Fibers by Pressurized Gyration for Reusable Face Masks. Polymers. 2022; 14(21):4498. https://doi.org/10.3390/polym14214498
Chicago/Turabian StyleHuang, Ruiran, Yanqi Dai, Jubair Ahmed, and Mohan Edirisinghe. 2022. "Facile One-Step Synthesis of PVDF Bead-on-String Fibers by Pressurized Gyration for Reusable Face Masks" Polymers 14, no. 21: 4498. https://doi.org/10.3390/polym14214498