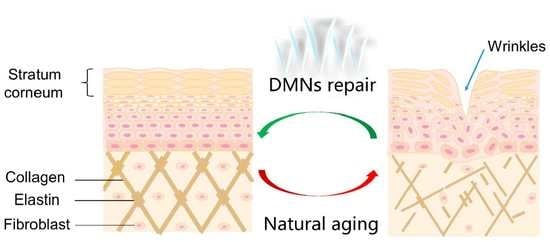
Design and Evaluation of Complex Polypeptide-Loaded Dissolving Microneedles for Improving Facial Wrinkles in Different Areas
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Volunteers
2.3. Preparation of CP-DMNs
2.4. Characterizations of CP-DMNs
2.5. In Vitro Safety Studies of CP-DMNs
2.6. In Vivo Skin Irritation Study of CP-DMNs
2.7. Clinical Studies of CP-DMNs
2.8. Statistical Analysis
3. Results and Discussion
3.1. Preparation and Characterization of DMNs
3.2. In Vitro and In Vivo Safety Studies of CP-DMNs
3.3. Clinical Studies of CP-DMNs vs. Placebo
3.4. Clinical Studies of CP-DMNs Applied at Different Areas
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Hänel, K.H.; Cornelissen, C.; Lüscher, B.; Baron, J.M. Cytokines and the Skin Barrier. Int. J. Mol. Sci. 2013, 14, 6720–6745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gunn, D.A.; Rexbye, H.; Griffiths, C.; Murray, P.G.; Fereday, A.; Catt, S.D.; Tomlin, C.C.; Strongitharm, B.H.; Perrett, D.; Catt, M.; et al. Why Some Women Look Young for Their Age. PLoS ONE 2009, 4, e8021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russell-Goldman, E.; Murphy, G.F. The Pathobiology of Skin Aging New Insights into an Old Dilemma. Am. J. Pathol. 2020, 190, 1356–1369. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.H.; Tiew, W.J.; Zhang, J.Y.; Ho, P.C.L.; Kachouie, N.N.; Kang, L.F. Geometrical optimisation of a personalised mi-croneedle eye patch for transdermal delivery of anti-wrinkle small peptide. Biofabrication 2020, 12, 035003. [Google Scholar] [CrossRef] [PubMed]
- Campione, E.; Cosio, T.; Lanna, C.; Mazzilli, S.; Dika, E.; Bianchi, L. Clinical efficacy, and reflectance confocal microscopy monitoring in moderate-severe skin aging treated with a polyvinyl gel containing retinoic and glycolic acid: An assessor-blinded 1-month study proof-of-concept trial. J. Cosmet. Dermatol. 2021, 20, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, S.; Shokri, J.; Ranjkesh, M.; Hamed, S.A.; Monajjemzadeh, F. Comparative physicochemical stability and clinical anti-wrinkle efficacy of transdermal emulgel preparations of 5% sodium ascorbyl phosphate and or ascorbic acid on human volunteers. J. Cosmet. Dermatol. 2020, 20, 174–180. [Google Scholar] [CrossRef]
- Ahsan, H. Immunopharmacology and immunopathology of peptides and proteins in personal products. J. Immunoass. Immunochem. 2019, 40, 439–447. [Google Scholar] [CrossRef]
- Ou, C.; Xu, X.; Wan, Y.; Xu, J.; Xia, X.; Ma, Y. Test and analysis of anti-aging effect of the eye cream containing polypeptides. China Surfactant Deterg. Cosmet. 2020, 50, 331–335. [Google Scholar]
- Wang, Y.; Wang, M.; Xiao, S.; Pan, P.; Li, P.; Huo, J. The Anti-Wrinkle Efficacy of Argireline, a Synthetic Hexapeptide, in Chinese Subjects. Am. J. Clin. Dermatol. 2013, 14, 147–153. [Google Scholar] [CrossRef]
- Kraeling, M.E.K.; Zhou, W.L.; Wang, P.; Ogunsola, O.A. In vitro skin penetration of acetyl hexapeptide-8 from a cosmetic formulation. Cutan. Ocul. Toxicol. 2015, 34, 46–52. [Google Scholar] [CrossRef]
- Avcil, M.; Akman, G.; Klokkers, J.; Jeong, D.; Çelik, A. Efficacy of bioactive peptides loaded on hyaluronic acid microneedle patches: A monocentric clinical study. J. Cosmet. Dermatol. 2019, 19, 328–337. [Google Scholar] [CrossRef]
- Lee, A.-R.C. Microneedle-mediated delivery of cosmeceutically relevant nucleoside and peptides in human skin: Challenges and strategies for dermal delivery. J. Pharm. Investig. 2019, 49, 587–601. [Google Scholar] [CrossRef]
- Choi, S.Y.; Kwon, H.J.; Ahn, G.R.; Ko, E.J.; Yoo, K.H.; Kim, B.J.; Lee, C.; Kim, D. Hyaluronic acid microneedle patch for the improvement of crow’s feet wrinkles. Dermatol. Ther. 2017, 30, e12546. [Google Scholar] [CrossRef]
- Kang, G.; Tu, T.N.T.; Kim, S.; Yang, H.; Jang, M.; Jo, D.; Ryu, J.; Baek, J.; Jung, H. Adenosine-loaded dissolving microneedle patches to improve skin wrinkles, dermal density, elasticity and hydration. Int. J. Cosmet. Sci. 2018, 40, 199–206. [Google Scholar] [CrossRef]
- Bukhari, S.N.A.; Roswandi, N.L.; Waqas, M.; Habib, H.; Hussain, F.; Khan, S.; Sohail, M.; Ramli, N.A.; Thu, H.E.; Hussain, Z. Hyaluronic acid, a promising skin rejuvenating biomedicine: A review of recent updates and pre-clinical and clinical investigations on cosmetic and nutricosmetic effects. Int. J. Biol. Macromol. 2018, 120, 1682–1695. [Google Scholar] [CrossRef]
- Saha, I.; Rai, V.K. Hyaluronic acid based microneedle array: Recent applications in drug delivery and cosmetology. Carbohydr. Polym. 2021, 267, 118168. [Google Scholar] [CrossRef]
- Albadr, A.A.; Tekko, I.A.; Vora, L.K.; Ali, A.A.; Laverty, G.; Donnelly, R.F.; Thakur, R.R.S. Rapidly dissolving microneedle patch of amphotericin B for intracorneal fungal infections. Drug Deliv. Transl. Res. 2021, 12, 931–943. [Google Scholar] [CrossRef]
- Xing, M.; Zhang, S.; Ma, Y.; Chen, Y.; Yang, G.; Zhou, Z.; Gao, Y. Preparation and evaluation of dissolving microneedle loaded with azelaic acid for acne vulgaris therapy. J. Drug Deliv. Sci. Technol. 2022, 75, 103667. [Google Scholar] [CrossRef]
- Xing, M.; Wang, X.; Zhao, L.; Zhou, Z.; Liu, H.; Wang, B.; Cheng, A.; Zhang, S.; Gao, Y. Novel dissolving microneedles preparation for synergistic melasma therapy: Combined effects of tranexamic acid and licorice extract. Int. J. Pharm. 2021, 600, 120406. [Google Scholar] [CrossRef]
- Zhu, M.; Liu, P.; Shi, H.G.; Tian, Y.; Ju, X.Y.; Jiang, S.D.; Li, Z.; Wu, M.; Niu, Z.W. Balancing antimicrobial activity with bio-logical safety: Bifunctional chitosan derivative for the repair of wounds with Gram-positive bacterial infections. Mater. Chem. B 2018, 6, 3884–3893. [Google Scholar] [CrossRef]
- Xing, M.Z.; Yang, G.Z.; Zhang, S.H.; Gao, Y.H. Acid-base combination principles for preparation of anti-acne dissolving mi-croneedles loaded with azelaic acid and matrine. Eur. J. Pharm. Sci. 2021, 165, 105935. [Google Scholar] [CrossRef] [PubMed]
- Indermun, S.; Luttge, R.; Choonara, Y.E.; Kumar, P.; Du Toit, L.C.; Modi, G.; Pillay, V. Current advances in the fabrication of microneedles for transdermal delivery. J. Control. Release 2014, 185, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.C.; Zhan, X.P.; Chen, S.J.; Mao, Z.M. Microneedles in Transdermal Drug Delivery System. Sci. Prog. 2007, 9, 385–390. [Google Scholar]
- Zhao, X.Y.; Zhang, S.H.; Yang, G.Z.; Zhou, Z.Q.; Gao, Y.H. Exploring Trehalose on the Release of Levonorgestrel from Implantable PLGA Microneedles. Polymer 2020, 12, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Z.; Zhang, S.; Yang, G.; Gao, Y. Enhanced delivery efficiency and sustained release of biopharmaceuticals by complexation-based gel encapsulated coated microneedles: rhIFNα-1b example. Asian J. Pharm. Sci. 2021, 16, 612–622. [Google Scholar] [CrossRef]
- Oikarinen, A. Aging of the skin connective tissue: How to measure the biochemical and mechanical properties of aging dermis. Photodermatol. Photoimmunol. Photomed. 1994, 10, 47–52. [Google Scholar]
- Mitzmacher, M.; Mithieux, S.; Weiss, A.; Hee, C.; Daniels, R. Novel Recombinant Tropoelastin Implants Restore Skin Extracellular Matrix. J. Drugs Dermatol. 2020, 19, 1166–1172. [Google Scholar] [CrossRef]
- Amano, S. Characterization and mechanisms of photoageing-related changes in skin. Damages of basement membrane and dermal structures. Exp. Dermatol. 2016, 25, 14–19. [Google Scholar] [CrossRef]
- Lim, S.H.; Kathuria, H.; Bin Amir, M.H.; Zhang, X.; Duong, H.T.; Ho, P.C.-L.; Kang, L. High resolution photopolymer for 3D printing of personalised microneedle for transdermal delivery of anti-wrinkle small peptide. J. Control. Release 2021, 329, 907–918. [Google Scholar] [CrossRef]
- Choi, Y.L.; Park, E.J.; Kim, E.; Na, D.H.; Shin, Y.-H. Dermal Stability and In Vitro Skin Permeation of Collagen Pentapeptides (KTTKS and palmitoyl-KTTKS). Biomol. Ther. 2014, 22, 321–327. [Google Scholar] [CrossRef] [Green Version]
- Yu, W.Q.; Hu, M.; Zhou, X.H.; Li, H.J. Preparation of oligopeptide and its application in daily chemical field. Detergent. Cos-Metics. 2013, 36, 28–34. [Google Scholar]
- El Hadmed, H.H.; Castillo, R.F. Cosmeceuticals: Peptides, proteins, and growth factors. J. Cosmet. Dermatol. 2016, 15, 514–519. [Google Scholar] [CrossRef]
- Jeong, S.; Yoon, S.; Kim, S.; Jung, J.; Kor, M.; Shin, K.; Lim, C.; Han, H.S.; Lee, H.; Park, K.-Y.; et al. Anti-Wrinkle Benefits of Peptides Complex Stimulating Skin Basement Membrane Proteins Expression. Int. J. Mol. Sci. 2020, 21, 73. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xing, M.; Liu, H.; Meng, F.; Ma, Y.; Zhang, S.; Gao, Y. Design and Evaluation of Complex Polypeptide-Loaded Dissolving Microneedles for Improving Facial Wrinkles in Different Areas. Polymers 2022, 14, 4475. https://doi.org/10.3390/polym14214475
Xing M, Liu H, Meng F, Ma Y, Zhang S, Gao Y. Design and Evaluation of Complex Polypeptide-Loaded Dissolving Microneedles for Improving Facial Wrinkles in Different Areas. Polymers. 2022; 14(21):4475. https://doi.org/10.3390/polym14214475
Chicago/Turabian StyleXing, Mengzhen, Han Liu, Fanda Meng, Yuning Ma, Suohui Zhang, and Yunhua Gao. 2022. "Design and Evaluation of Complex Polypeptide-Loaded Dissolving Microneedles for Improving Facial Wrinkles in Different Areas" Polymers 14, no. 21: 4475. https://doi.org/10.3390/polym14214475