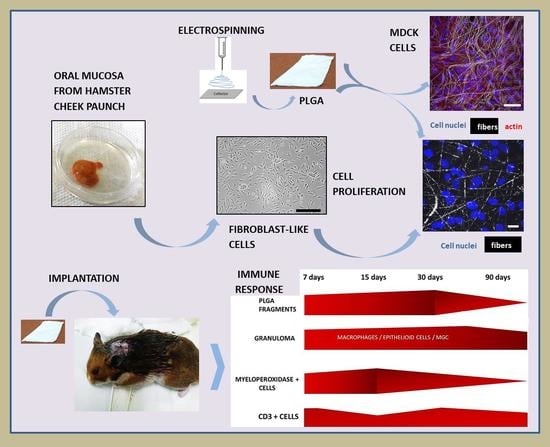
In Vitro and In Vivo Cell-Interactions with Electrospun Poly (Lactic-Co-Glycolic Acid) (PLGA): Morphological and Immune Response Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. PLGA
2.2. Electrospinning Process
2.3. In Vitro Experiments
2.3.1. MDCK Cells Cultured onto PLGA Membranes
2.3.2. Primary Hamsters’ Fibroblasts Cultured onto PLGA Membranes
2.4. In Vivo Experiment
2.5. PLGA Implantation and Material Collection
2.6. Sample Preparation for Morphological Analyzes by Light and Electron Microscopies
2.7. Immunohistochemistry
2.8. Morphometrical and Statistical Analysis
3. Results
3.1. In Vitro Experiments
3.1.1. MDCK-Cell Line
3.1.2. In Vitro Experiment Using Primary Fibroblast-like Cells
3.2. In Vivo Experiment
3.2.1. Histopathology
3.2.2. Immunohistochemistry and Morphometry
3.2.3. Transmission Electron Microscopy (TEM)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gupta, B.; Revagade, N.; Hilborn, J. Poly(Lactic Acid) Fiber: An Overview. Prog. Polym. Sci. 2007, 32, 455–482. [Google Scholar] [CrossRef]
- Zafar, M.; Najeeb, S.; Khurshid, Z.; Vazirzadeh, M.; Zohaib, S.; Najeeb, B.; Sefat, F. Potential of Electrospun Nanofibers for Biomedical and Dental Applications. Materials 2016, 9, 73. [Google Scholar] [CrossRef] [PubMed]
- Hughes, G.A. Nanostructure-Mediated Drug Delivery. In Nanomedicine in Cancer; Balogh, L.P., Ed.; Pan Stanford Publishing: Singapore, 2017; pp. 47–72. [Google Scholar]
- Elmowafy, E.M.; Tiboni, M.; Soliman, M.E. Biocompatibility, Biodegradation and Biomedical Applications of Poly (Lactic Acid)/Poly (Lactic-Co-Glycolic Acid) Micro and Nanoparticles. J. Pharm. Investig. 2019, 49, 347–380. [Google Scholar] [CrossRef]
- Adusei, K.M.; Ngo, T.B.; Sadtler, K. T Lymphocytes as Critical Mediators in Tissue Regeneration, Fibrosis, and the Foreign Body Response. Acta Biomater. 2021, 133, 17–33. [Google Scholar] [CrossRef]
- Soni, S.S.; Rodell, C.B. Polymeric Materials for Immune Engineering: Molecular Interaction to Biomaterial Design. Acta Biomater. 2021, 133, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Xu, C.; Wu, G.; Ye, Q.; Wang, C. Poly(Lactic-co-Glycolic Acid): Applications and Future Prospects for Periodontal Tissue Regeneration. Polymers 2017, 9, 189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, A.T.C.R.; Cardoso, B.C.O.; e Silva, M.E.S.R.; Freitas, R.F.S.; Sousa, R.G. Synthesis, Characterization, and Study of PLGA Copolymer in Vitro Degradation. J. Biomater. Nanobiotechnol. 2015, 06, 8–19. [Google Scholar] [CrossRef] [Green Version]
- Zafar, M.S.; Khurshid, Z.; Almas, K. Oral Tissue Engineering Progress and Challenges. Tissue Eng. Regen. Med. 2015, 12, 387–397. [Google Scholar] [CrossRef]
- Stratton, S.; Shelke, N.B.; Hoshino, K.; Rudraiah, S.; Kumbar, S.G. Bioactive Polymeric Scaffolds for Tissue Engineering. Bioact. Mater. 2016, 1, 93–108. [Google Scholar] [CrossRef]
- Lobo, A.O.; Afewerki, S.; de Paula, M.M.M.; Ghannadian, P.; Marciano, F.R.; Zhang, Y.S.; Webster, T.J.; Khademhosseini, A. Electrospun Nanofiber Blend with Improved Mechanical and Biological Performance. Int. J. Nanomed. 2018, 13, 7891–7903. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Zhang, X.; Liu, N.; Yu, X.; Gao, M.; Wang, W.; Wu, T. Engineering Electrospun Nanofibers for the Treatment of Oral Diseases. Front. Chem. 2021, 9, 797523. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.L.; Dip, R.M.; Souza, D.H.; Nascimento, J.P.; Santos, A.P.; Furtado, C.A. Electrospun Nanofibers of Poly (Lactic Acid)/Graphene Nanocomposites. J. Nanosci. Nanotechnol. 2017, 17, 2531–2540. [Google Scholar] [CrossRef] [PubMed]
- Bye, F.J.; Wang, L.; Bullock, A.; Blackwood, K.A.; Ryan, A.; MacNeil, S. Postproduction Processing of Electrospun Fibres for Tissue Engineering. J. Vis. Exp. 2012, 66, e4172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chor, A.; Gonçalves, R.; Costa, A.; Farina, M.; Ponche, A.; Sirelli, L.; Schrodj, G.; Gree, S.; Andrade, L.; Anselme, K.; et al. In Vitro Degradation of Electrospun Poly(Lactic-Co-Glycolic Acid) (PLGA) for Oral Mucosa Regeneration. Polymers 2020, 12, 1853. [Google Scholar] [CrossRef]
- Liang, Y.; Liang, Y.; Zhang, H.; Guo, B. Antibacterial Biomaterials for Skin Wound Dressing. Asian J. Pharm. Sci. 2022, 17, 353–384. [Google Scholar] [CrossRef]
- Wells, E.K.; Iii, O.Y.; Lifton, R.P.; Cantley, L.G.; Caplan, M.J. Epithelial Morphogenesis of MDCK Cells in Three-Dimensional Collagen Culture Is Modulated by Interleukin-8. Am. J. Physiol. Physiol. 2013, 304, C966–C975. [Google Scholar] [CrossRef] [Green Version]
- Chor, A.; Skeff, M.A.; Takiya, C.; Gonçalves, R.; Dias, M.; Farina, M.; Andrade, L.R.; Coelho, V.D.M. Emerging Approaches of Wound Healing in Experimental Models of High-Grade Oral Mucositis Induced by Anticancer Therapy. Oncotarget 2021, 12, 2283–2299. [Google Scholar] [CrossRef]
- Makadia, H.K.; Siegel, S.J. Poly Lactic-Co-Glycolic Acid (PLGA) As Biodegradable Controlled Drug Delivery Carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef]
- Kim, M.S.; Ahn, H.H.; Na Shin, Y.; Cho, M.H.; Khang, G.; Lee, H.B. An In Vivo Study of the Host Tissue Response to Subcutaneous Implantation of PLGA- and/or Porcine Small Intestinal Submucosa-Based Scaffolds. Biomaterials 2007, 28, 5137–5143. [Google Scholar] [CrossRef]
- Ramot, Y.; Touitou, D.; Levin, G.; Ickowicz, D.E.; Zada, M.H.; Abbas, R.; Domb, A.; Nyska, A. Interspecies Differences in Reaction to a Biodegradable Subcutaneous Tissue Filler: Severe Inflammatory Granulomatous Reaction in the Sinclair Minipig. Toxicol. Pathol. 2015, 43, 267–271. [Google Scholar]
- Ramot, Y.; Haim-Zada, M.; Domb, A.J.; Nyska, A. Biocompatibility and Safety of PLA and Its Copolymers. Adv. Drug Deliv. Rev. 2016, 107, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Cao, Z.; Bai, T.; Carr, L.R.; Ella-Menye, J.-R.; Irvin, C.; Ratner, B.D.; Jiang, S. Zwitterionic Hydrogels Implanted in Mice Resist the Foreign-Body Reaction. Nat. Biotechnol. 2013, 31, 553–556. [Google Scholar] [CrossRef]
- Milleret, V.; Simona, B.; Neuenschwander, P.; Hall, H. Tuning Electrospinning Parameters for Production of 3D-Fiber-Fleeces with Increased Porosity for Soft Tissue Engineering Applications. Eur. Cells Mater. 2011, 21, 286–303. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.M.; Rodriguez, A.; Chang, D.T. Foreign Body Reaction to Biomaterials. Semin. Immunol. 2008, 20, 86–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, J.M. In Vitro and In Vivo Monocyte, Macrophage, Foreign Body Giant Cell, and Lymphocyte Interactions with Biomaterials. In Biological Interactions on Materials Surfaces; Springer: New York, NY, USA, 2009; Volume 1, pp. 225–244. [Google Scholar]
- Mcnally, A.K.; Anderson, J.M. Macrophage Fusion and Multinucleated Giant Cells of Inflammation. In Cell Fusion in Health and Disease; Dittmar, T., Zänker, K.S., Eds.; Springer: Dordrecht, The Netherlands, 2011; Volume 713. [Google Scholar]
- Rediguieri, C.F.; Sassonia, R.C.; Dua, K.; Kikuchi, I.S.; Pinto, T.D.J.A. Impact of Sterilization Methods on Electrospun Scaffolds for Tissue Engineering. Eur. Polym. J. 2016, 82, 181–195. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumbar, S.G.; Nukavarapu, S.P.; James, R.; Nair, L.S.; Laurencin, C.T. Electrospun Poly(Lactic Acid-Co-Glycolic Acid) Scaffolds For Skin Tissue Engineering. Biomaterials 2008, 29, 4100–4107. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.H.; Park, J.H.; El-Fiqi, A.; Kim, J.H.; Yun, Y.R.; Jang, J.H.; Han, C.M.; Lee, E.J.; Kim, H.W. Biointerface Control of Electrospun Fiber Scaffolds for Bone Regeneration: Engineered Protein Link to Mineralized Surface. Acta Biomater. 2014, 10, 2750–2761. [Google Scholar] [CrossRef]
- Li, L.; Zhou, G.; Wang, Y.; Yang, G.; Ding, S.; Zhou, S. Controlled Dual Delivery Of BMP-2 and Dexamethasone by Nanoparticle-Embedded Electrospun Nanofibers for the Efficient Repair of Critical-Sized Rat Calvarial Defect. Biomaterials 2015, 37, 218–229. [Google Scholar] [CrossRef]
- Zhang, X.; Shi, X.; Gautrot, J.E.; Peijs, T. Nanoengineered Electrospun Fibers and Their Biomedical Applications: A Review. Nanocomposites 2020, 7, 1–34. [Google Scholar] [CrossRef]
- Chou, S.-F.; Carson, D.; Woodrow, K.A. Current Strategies for Sustaining Drug Release from Electrospun Nanofibers. J. Control. Release 2015, 220, 584–591. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, L.E.; Zegers, M.M.; Mostov, K.E. Building Epithelial Architecture: Insights from Three-Dimensional Culture Models. Nat. Rev. Mol. Cell Biol. 2002, 3, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Duan, Z.; Liu, S.; Pachon, I.; Ma, J.; Hemstreet, G.P.; Zhang, Y. Drug-Induced Nephrotoxicity Assessment in 3D Cellular Models. Micromachines 2021, 13, 3. [Google Scholar] [CrossRef] [PubMed]
- Bhaskar, P.; Bosworth, L.A.; Wong, R.; O’brien, M.A.; Kriel, H.; Smit, E.; McGrouther, D.A.; Wong, J.K.; Cartmell, S.H. Cell Response to Sterilized Electrospun Poly (Ɛ-Caprolactone) Scaffolds to Aid Tendon Regeneration In Vivo. J. Biomed. Mater. Res. Part A 2017, 105, 389–397. [Google Scholar] [CrossRef]
- Hakki, S.S.; Korkusuz, P.; Purali, N.; Bozkurt, B.; Kuş, M.; Duran, I. Attachment, Proliferation and Collagen Type I Mrna Expression of Human Gingival Fibroblasts on Different Biodegradable Membranes. Connect. Tissue Res. 2013, 54, 260–266. [Google Scholar] [CrossRef]
- Campos, D.M.; Gritsch, K.; Salles, V.; Attik, G.N.; Grosgogeat, B. Surface Entrapment of Fibronectin on Electrospun PLGA Scaffolds for Periodontal Tissue Engineering. BioRes. Open Access 2014, 3, 117–126. [Google Scholar] [CrossRef]
- Sadeghi, A.R.; Nokhasteh, S.; Molavi, A.M.; Khorsand-Ghayeni, M.; Naderi-Meshkin, H.; Mahdizadeh, A. Surface Modification of Electrospun PLGA Scaffold with Collagen for Bioengineered Skin Substitutes. Mater. Sci. Eng. C 2016, 66, 130–137. [Google Scholar] [CrossRef]
- Sadeghi-Avalshahr, A.R.; Khorsand-Ghayeni, M.; Nokhasteh, S.; Molavi, A.M.; Naderi-Meshkin, H. Synthesis and Characterization of PLGA/Collagen Composite Scaffolds as Skin Substitute Produced by Electrospinning Through Two Different Approaches. J. Mater. Sci. Mater. Med. 2017, 28, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Helling, A.L.; Viswanathan, P.; Cheliotis, K.S.; Mobasseri, S.A.; Yang, Y.; El Haj, A.J.; Watt, F.M. Dynamic Culture Substrates That Mimic the Topography of the Epidermal–Dermal Junction. Tissue Eng. Part A 2019, 25, 214–223. [Google Scholar] [CrossRef] [Green Version]
- Inanç, B.; Arslan, Y.E.; Seker, S.; Elçin, A.E.; Elçin, Y.M. Periodontal Ligament Cellular Structures Engineered With Electrospun Poly(DL-Lactide-Co-Glycolide) Nanofibrous Membrane Scaffolds. J. Biomed. Mater. Res. Part A 2009, 90, 186–195. [Google Scholar] [CrossRef]
- Lowery, J.L.; Datta, N.; Rutledge, G.C. Effect of Fiber Diameter, Pore Size and Seeding Method on Growth of Human Dermal Fibroblasts in Electrospun Poly (Ɛ-Caprolactone) Fibrous Mats. Biomaterials 2010, 31, 491–504. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.M. Biological Responses to Materials. Annu. Rev. Mater. Sci. 2001, 31, 81–110. [Google Scholar] [CrossRef]
- Ratner, B.D. Reducing Capsular Thickness and Enhancing Angiogenesis around Implant Drug Release Systems. J. Control. Release 2001, 78, 211–218. [Google Scholar] [CrossRef]
- Balabiyev, A.; Podolnikova, N.P.; Kilbourne, J.A.; Baluch, D.P.; Lowry, D.; Zare, A.; Ros, R.; Flick, M.J.; Ugarova, T.P. Fibrin Polymer on the Surface of Biomaterial Implants Drives the Foreign Body Reaction. Biomaterials 2021, 277, 121087. [Google Scholar] [CrossRef] [PubMed]
- Ward, W.K. A Review of the Foreign-body Response to Subcutaneously-implanted Devices: The Role of Macrophages and Cytokines in Biofouling and Fibrosis. J. Diabetes Sci. Technol. 2008, 2, 768–777. [Google Scholar] [CrossRef] [Green Version]
- Anderson, J.M.; Cramer, S. Perspectives on the Inflammatory, Healing, and Foreign Body Responses to Biomaterials and Medical Devices. In Host Response to Biomaterials in Chapter 2; Academic Press: Cambridge, MA, USA, 2015; pp. 13–36. ISBN 9780128001967. [Google Scholar] [CrossRef]
- Böstman, O.; Hirvensalo, E.; Vainionpää, S.; Mäkelä, A.; Vihtonen, K.; Törmälä, P.; Rokkanen, P. Ankle Fractures Treated Using Biodegradable Internal Fixation. Clin. Orthop. Relat. Res. 1989, 138, 195–203. [Google Scholar] [CrossRef]
- Hirvensalo, E. Fracture Fixation with Biodegradable Rods Forty-One Cases of Severe Ankle Fractures. Acta Orthop. Scand. 1989, 60, 601–606. [Google Scholar] [CrossRef]
- Bostman, O.; Hirvensalo, E.; Makinen, J.; Rokkanen, P. Foreign-Body Reactions to Fracture Fixation Implants of Biodegradable Synthetic Polymers. J. Bone Jt. Surgery. Br. Vol. 1990, 72-B, 592–596. [Google Scholar] [CrossRef] [Green Version]
- Poigenfürst, J.; Leixnering, M.; Ben Mokhtar, M. Lokalkomplikationen Nach Implantation von Biorod. Aktuelle Traumatol. 1990, 20, 157–159. [Google Scholar]
- Bostman, O. Osteolytic Changes Accompanying Degradation of Absorbable Fracture Fixation Implants. J. Bone Jt. Surgery. Br. Vol. 1991, 73-B, 679–682. [Google Scholar] [CrossRef]
- Chambers, T.J. Multinucleate Giant Cells. J. Pathol. 1978, 126, 125–148. [Google Scholar] [CrossRef] [PubMed]
- Helming, L.; Gordon, S. The Molecular Basis of Macrophage Fusion. Immunobiology 2008, 212, 785–793. [Google Scholar] [CrossRef]
- Xia, Z.D.; Zhu, T.B.; Du, J.Y.; Zheng, Q.X.; Wang, L.; Li, S.P.; Chang, C.Y.; Fang, S.Y. Macrophages in Degradation of Collagen/Hydroxylapatite (CHA), Beta-Tricalcium Phosphate Ceramics (TCP) Artificial Bone Graft: An In Vivo Study. Chin. Med. J. 1994, 107, 845–849. [Google Scholar]
- Christenson, E.M.; Anderson, J.M.; Hiltner, A. Oxidative Mechanisms of Poly (Carbonate Urethane) and Poly (Ether Urethane) Biodegradation: In Vivo and In Vitro correlations. J. Biomed. Mater. Res. Part A Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2004, 70, 245–255. [Google Scholar] [CrossRef]
- Santerre, J.; Woodhouse, K.; Laroche, G.; Labow, R. Understanding the Biodegradation of Polyurethanes: From Classical Implants to Tissue Engineering Materials. Biomaterials 2005, 26, 7457–7470. [Google Scholar] [CrossRef]
- Kim, Y.K.; Chen, E.Y.; Liu, W.F. Biomolecular Strategies to Modulate the Macrophage Response to Implanted Materials. J. Mater. Chem. B 2015, 4, 1600–1609. [Google Scholar] [CrossRef]
- McNally, A.K.; Jones, J.A.; MacEwan, S.; Colton, E.; Anderson, J.M. Vitronectin is a Critical Protein Adhesion Substrate For IL-4-Induced Foreign Body Giant Cell Formation. J. Biomed. Mater. Res. Part A 2007, 86, 535–543. [Google Scholar] [CrossRef]
- Van der Rhee, H.J.; Winter, C.P.M.V.D.B.-D.; Daems, W.T. The Differentiation of Monocytes into Macrophages, Epithelioid Cells, And Multinucleated Giant Cells in Subcutaneous Granulomas. Cell Tissue Res. 1979, 197, 355–378. [Google Scholar] [CrossRef]
- Franz, S.; Rammelt, S.; Scharnweber, D.; Simon, J.C. Immune Responses to Implants—A Review of The Implications for the Design of Immunomodulatory Biomaterials. Biomaterials 2011, 32, 6692–6709. [Google Scholar] [CrossRef]
- Gentile, P.; Chiono, V.; Carmagnola, I.; Hatton, P.V. An Overview of Poly(lactic-co-glycolic) Acid (PLGA)-Based Biomaterials for Bone Tissue Engineering. Int. J. Mol. Sci. 2014, 15, 3640–3659. [Google Scholar] [CrossRef]
- Yamashiro, S.; Kamohara, H.; Wang, J.M.; Yang, D.; Gong, W.H.; Yoshimura, T. Phenotypic and Functional Change of Cytokine-Activated Neutrophils: Inflammatory Neutrophils Are Heterogeneous and Enhance Adaptive Immune Responses. J. Leukoc. Biol. 2001, 69, 698–704. [Google Scholar] [CrossRef]
- Gilroy, D.W. The Endogenous Control of Acute Inflammation–from Onset to Resolution. Drug Discov. Today: Ther. Strateg. 2004, 1, 313–319. [Google Scholar] [CrossRef]
- Brodbeck, W.G.; MacEwan, M.; Colton, E.; Meyerson, H.; Anderson, J.M. Lymphocytes and the Foreign Body Response: Lymphocyte Enhancement of Macrophage Adhesion and Fusion. J. Biomed. Mater. Res. Part A 2005, 74A, 222–229. [Google Scholar] [CrossRef]
- Barbeck, M.; Udeabor, S.; Lorenz, J.; Schlee, M.; Holthaus, M.G.; Raetscho, N.; Choukroun, J.; Sader, R.; Kirkpatrick, C.J.; Ghanaati, S. High-Temperature Sintering of Xenogeneic Bone Substitutes Leads to Increased Multinucleated Giant Cell Formation: In Vivo and Preliminary Clinical Results. J. Oral Implant. 2015, 41, e212–e222. [Google Scholar] [CrossRef]
- Ghanaati, S.; Schlee, M.; Webber, M.J.; Willershausen, I.; Barbeck, M.; Balic, E.; Görlach, C.; Stupp, S.I.; Sader, R.A.; Kirkpatrick, C.J. Evaluation of the Tissue Reaction to a New Bilayered Collagen Matrix In Vivo And Its Translation to the Clinic. Biomed. Mater. 2011, 6, 015010. [Google Scholar] [CrossRef]
- Ghanaati, S.; Barbeck, M.; Detsch, R.; Deisinger, U.; Hilbig, U.; Rausch, V.; Sader, R.; Unger, R.E.; Ziegler, G.; Kirkpatrick, C.J. The Chemical Composition of Synthetic Bone Substitutes Influences Tissue Reactions In Vivo: Histological and Histomorphometrical Analysis of the Cellular Inflammatory Response to Hydroxyapatite, Beta-Tricalcium Phosphate and Biphasic Calcium Phosphate Ceramics. Biomed. Mater. 2012, 7, 015005. [Google Scholar]
- Klopfleisch, R.; Jung, F. The Pathology of the Foreign Body Reaction against Biomaterials. J. Biomed. Mater. Res. Part A 2016, 105, 927–940. [Google Scholar] [CrossRef]
- Blackwood, K.A.; McKean, R.; Canton, I.; Freeman, C.O.; Franklin, K.L.; Cole, D.; Brook, I.; Farthing, P.; Rimmer, S.; Haycock, J.W.; et al. Development of Biodegradable Electrospun Scaffolds for Dermal Replacement. Biomaterials 2008, 29, 3091–3104. [Google Scholar] [CrossRef]
- Anderson, J.M. Exploiting the Inflammatory Response on Biomaterials Research and Development. J. Mater. Sci. Mater. Electron. 2015, 26, 1–2. [Google Scholar] [CrossRef]
- Zandstra, J.; Hiemstra, C.; Petersen, A.; Zuidema, J.; van Beuge, M.; Rodriguez, S.; Lathuile, A.; Veldhuis, G.; Steendam, R.; Bank, R.; et al. Microsphere Size Influences the Foreign Body Reaction. Eur. Cells Mater. 2014, 28, 335–347. [Google Scholar] [CrossRef]
- Mitragotri, S.; Lahann, J. Physical Approaches to Biomaterial Design. Nat. Mater. 2009, 8, 15–23. [Google Scholar] [CrossRef] [Green Version]
- Lu, L.; Peter, S.J.; Lyman, M.D.; Lai, H.-L.; Leite, S.M.; Tamada, J.A.; Uyama, S.; Vacanti, J.P.; Langer, R.; Mikos, A.G. In Vitro and In Vivo Degradation of Porous Poly(Dl-Lactic-Co-Glycolic Acid) Foams. Biomaterials 2000, 21, 1837–1845. [Google Scholar] [CrossRef]
- Kaushiva, A.; Turzhitsky, V.M.; Backman, V.; Ameer, G.A. A Biodegradable Vascularizing Membrane: A Feasibility Study. Acta Biomater. 2007, 3, 631–642. [Google Scholar] [CrossRef]
- Whitaker, R.; Hernaez-Estrada, B.; Hernandez, R.M.; Santos-Vizcaino, E.; Spiller, K.L. Immunomodulatory Biomaterials for Tissue Repair. Chem. Rev. 2021, 121, 11305–11335. [Google Scholar] [CrossRef]
- Al-Maawi, S.; Orlowska, A.; Sader, R.; Kirkpatrick, C.J.; Ghanaati, S. In Vivo Cellular Reactions to Different Biomaterials-Physiological and Pathological Aspects and Their Consequences. Semin. Immunol. 2017, 29, 49–61. [Google Scholar] [CrossRef]
- Madden, L.R.; Mortisen, D.J.; Sussman, E.M.; Dupras, S.K.; Fugate, J.A.; Cuy, J.L.; Hauch, K.D.; Laflamme, M.A.; Murry, C.E.; Ratner, B.D. Proangiogenic Scaffolds as Functional Templates for Cardiac Tissue Engineering. Proc. Natl. Acad. Sci. USA 2010, 107, 15211–15216. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Zhou, X.; Shen, Y.; Li, H.; Zhou, G.; Zhang, W.; Zhang, Y.; Liu, W. Asiaticoside Loading into Polylactic-Co-Glycolic Acid Electrospun Nanofibers Attenuates Host Inflammatory Response and Promotes M2 Macrophage Polarization. J. Biomed. Mater. Res. Part A 2019, 108, 69–80. [Google Scholar] [CrossRef]
- Lee, Y.; Kwon, J.; Khang, G.; Lee, D. Reduction of Inflammatory Responses and Enhancement of Extracellular Matrix Formation by Vanillin-Incorporated Poly (Lactic-Co-Glycolic Acid) Scaffolds. Tissue Eng. Part A 2012, 18, 1967–1978. [Google Scholar] [CrossRef] [Green Version]
- Adabi, M.; Naghibzadeh, M.; Adabi, M.; Zarrinfard, M.A.; Esnaashari, S.S.; Seifalian, A.M.; Faridi-Majidi, R.; Aiyelabegan, H.T.; Ghanbari, H. Biocompatibility and Nanostructured Materials: Applications in Nanomedicine. Artif. Cells Nanomed. Biotechnol. 2017, 45, 833–842. [Google Scholar] [CrossRef]
- Sonis, S.T.; Tracey, C.; Shklar, G.; Jenson, J.; Florine, D. An Animal Model for Mucositis Induced by Cancer Chemotherapy. Oral Surg. Oral Med. Oral Pathol. 1990, 69, 437–443. [Google Scholar] [CrossRef]
- Tanideh, N.; Tavakoli, P.; Saghiri, M.A.; Garcia-Godoy, F.; Amanat, D.; Tadbir, A.A.; Samani, S.M.; Tamadon, A. Healing Acceleration in Hamsters of Oral Mucositis Induced by 5-Fluorouracil with Topical Calendula Officinalis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2013, 115, 332–338. [Google Scholar] [CrossRef]
- Ribeiro, S.B.; De Araújo, A.A.; Araújo Júnior, R.F.; Brito, G.A.C.; Leitão, R.C.; Barbosa, M.M.; Garcia, V.B.; Medeiros, A.C.; Medeiros, C.A.C.X. Protective Effect of Dexamethasone on 5-FU-Induced Oral Mucositis in Hamsters. PLoS ONE 2017, 12, E0186511. [Google Scholar] [CrossRef]
- Jung, H.; Kim, H.S.; Lee, J.H.; Lee, J.J.; Park, H.S. Wound Healing Promoting Activity of Tonsil-Derived Stem Cells on 5-Fluorouracil-Induced Oral Mucositis Model. Tissue Eng. Regen. Med. 2020, 17, 105–119. [Google Scholar] [CrossRef]
- Warner, B.M.; Safronetz, D.; Kobinger, G.P. Syrian Hamsters as a Small Animal Model for Emerging Infectious Diseases: Advances in Immunologic Methods. In Emerging and Re-Emerging Viral Infections; Springer: Cham, Switzerland, 2016; pp. 87–101. [Google Scholar]






| Antibody | Manufacturer | Antigenic Recovery | Dilution |
|---|---|---|---|
| CD3 | Dako, polyclonal rabbit, cat.# GA503 | Steamer 20 min, Citrate Buffer 0.01 M pH 6.0 | 1: 400 |
| Myeloperoxidase | AbCam, CA, USA, polyclonal rabbit, cat. # ab9535 | Microwave—3 min 3 x, Citrate Buffer 0.01 M pH 6.0 | 1:100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chor, A.; Takiya, C.M.; Dias, M.L.; Gonçalves, R.P.; Petithory, T.; Cypriano, J.; de Andrade, L.R.; Farina, M.; Anselme, K. In Vitro and In Vivo Cell-Interactions with Electrospun Poly (Lactic-Co-Glycolic Acid) (PLGA): Morphological and Immune Response Analysis. Polymers 2022, 14, 4460. https://doi.org/10.3390/polym14204460
Chor A, Takiya CM, Dias ML, Gonçalves RP, Petithory T, Cypriano J, de Andrade LR, Farina M, Anselme K. In Vitro and In Vivo Cell-Interactions with Electrospun Poly (Lactic-Co-Glycolic Acid) (PLGA): Morphological and Immune Response Analysis. Polymers. 2022; 14(20):4460. https://doi.org/10.3390/polym14204460
Chicago/Turabian StyleChor, Ana, Christina Maeda Takiya, Marcos Lopes Dias, Raquel Pires Gonçalves, Tatiana Petithory, Jefferson Cypriano, Leonardo Rodrigues de Andrade, Marcos Farina, and Karine Anselme. 2022. "In Vitro and In Vivo Cell-Interactions with Electrospun Poly (Lactic-Co-Glycolic Acid) (PLGA): Morphological and Immune Response Analysis" Polymers 14, no. 20: 4460. https://doi.org/10.3390/polym14204460