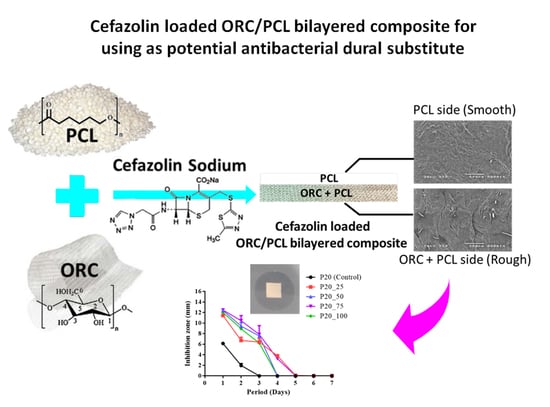
Cefazolin Loaded Oxidized Regenerated Cellulose/Polycaprolactone Bilayered Composite for Use as Potential Antibacterial Dural Substitute
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Sample Preparation
2.3. Microstructure
2.4. Bulk Density
2.5. Tensile Properties
2.6. Total Cefazolin Loading Content
2.7. In Vitro Cefazolin Release Study
2.8. Minimum Inhibitory Concentration (MIC)
2.9. Antibacterial Activity
2.10. Statistical Analysis
3. Results
3.1. Physical and Mechanical Properties
3.1.1. Microstructure, Bulk Density and Thickness
3.1.2. Tensile Properties
3.2. Total Cefazolin Content
3.3. In Vitro Cefazolin Release
3.4. Minimum Inhibitory Concentration (MIC)
3.5. Antibacterial Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, W.; Ao, Q. Research and application progress on dural substitutes. J. Neurorestoratol. 2019, 7, 161–170. [Google Scholar] [CrossRef] [Green Version]
- Choi, E.H.; Chan, A.Y.; Brown, N.J.; Lien, B.V.; Sahyouni, R.; Chan, A.K.; Roufail, J.; Oh, M.Y. Effectiveness of repair techniques for spinal dural tears: A systematic review. World Neurosurg. 2021, 149, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Suwanprateeb, J.; Luangwattanawilai, T.; Theeranattapong, T.; Suvannapruk, W.; Sorayouth, C.; Hemstapat, W. Bilayer oxidized regenerated cellulose/poly ε-caprolactone knitted fabric-reinforced composite for use as an artificial dural substitute. J. Mater. Sci. Mater. Med. 2016, 27, 122–134. [Google Scholar] [CrossRef] [PubMed]
- Hemstapat, R.; Suvannapruk, W.; Thammarakcharoen, F.; Chumnanvej, S.; Suwanprateeb, J. Performance evaluation of bilayer oxidized regenerated cellulose/poly ε-caprolactone knitted fabric reinforced composites for dural substitution. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2020, 234, 854–863. [Google Scholar] [CrossRef]
- Chumnanvej, S.; Luangwattanawilai, T.; Rawiwet, V.; Suwanprateeb, J.; Rattanapinyopituk, K.; Huaijantug, S.; Yinharnmingmongkol, C.; Hemstapat, R. In vivo evaluation of bilayer ORC/PCL composites in a rabbit model for using as a dural substitute. Neurol. Res. 2020, 42, 879–889. [Google Scholar] [CrossRef]
- Dwivedi, R.; Kumar, S.; Pandey, R.; Mahajan, A.; Nandana, D.; Katti, D.S.; Mehrotra, D. Polycaprolactone as biomaterial for bone scaffolds: Review of literature. J. Oral Biol. Craniofac. Res. 2020, 10, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Backes, E.H.; Harb, S.V.; Beatrice, C.A.G.; Shimomura, K.M.B.; Passador, F.R.; Costa, L.C.; Pessan, L.A. Polycaprolactone usage in additive manufacturing strategies for tissue engineering applications: A review. J. Biomed. Mater. Res. B Appl. Biomater. 2022, 110, 1479–1503. [Google Scholar] [CrossRef] [PubMed]
- Kleine, J.; Leisz, S.; Ghadban, C.; Hohmann, T.; Prell, J.; Scheller, C.; Strauss, C.; Simmermacher, S.; Dehghani, F. Variants of oxidized regenerated cellulose and their distinct effects on neuronal tissue. Int. J. Mol. Sci. 2021, 22, 11467. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, M.; Righi, E.; Astilean, A.; Corcione, S.; Petrolo, A.; Farina, E.C.; De Rosa, F.G. Antimicrobial prophylaxis in minor and major surgery. Minerva Anestesiol. 2015, 81, 76–91. [Google Scholar] [PubMed]
- Erman, T.; Demirhindi, H.; Göçer, A.İ.; Tuna, M.; İldan, F.; Boyar, B. Risk factors for surgical site infections in neurosurgery patients with antibiotic prophylaxis. Surg. Neurol. 2005, 63, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Bratzler, D.W.; Dellinger, E.P.; Olsen, K.M.; Perl, T.M.; Auwaerter, P.G.; Bolon, M.K.; Fish, D.N.; Napolitano, L.M.; Sawyer, R.G.; Slain, D.; et al. Clinical practice guidelines for antimicrobial prophylaxis in surgery. Am. J. Health Syst. Pharm. 2013, 70, 195–283. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Ni, M.; Zhang, Y.; Groen, R.J. Antibiotic prophylaxis in craniotomy: A review. Neurosurg. Rev. 2014, 37, 407–414. [Google Scholar] [CrossRef]
- Gyssens, I.C. Preventing postoperative infections: Current treatment recommendations. Drugs 1999, 57, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Brocard, E.; Reveiz, L.; Régnaux, J.P.; Abdala, V.; Ramón-Pardo, P.; del Rio Bueno, A. Antibiotic prophylaxis for surgical procedures: A scoping review. Rev. Panam. Salud. Publica 2021, 45, e62. [Google Scholar] [CrossRef] [PubMed]
- Kusaba, T. Safety and efficacy of cefazolin sodium in the management of bacterial infection and in surgical prophylaxis. Clin. Med. Ther. 2009, 1, 1607–1615. [Google Scholar] [CrossRef] [Green Version]
- Wassif, R.K.; Elkayal, M.; Shamma, R.N.; Elkheshen, S.A. Recent advances in the local antibiotics delivery systems for management of osteomyelitis. Drug Deliv. 2021, 28, 2392–2414. [Google Scholar] [CrossRef] [PubMed]
- Stebbins, N.D.; Ouimet, M.A.; Uhrich, K.E. Antibiotic-containing polymers for localized, sustained drug delivery. Adv. Drug Deliv. Rev. 2014, 78, 77–87. [Google Scholar] [CrossRef] [Green Version]
- Dash, A.K.; Cudworth, G.C. Therapeutic applications of implantable drug delivery systems. J. Pharmacol. Toxicol. Methods 1998, 40, 1–12. [Google Scholar] [CrossRef]
- Wu, P.; Grainger, D.W. Drug/device combinations for local drug therapies and infection prophylaxis. Biomaterials 2006, 27, 2450–2467. [Google Scholar] [CrossRef] [PubMed]
- Mutsuzaki, H.; Oyane, A.; Sogo, Y.; Sakane, M.; Ito, A. Cefazolin-containing poly(ε-caprolactone) sponge pad to reduce pin tract infection rate in rabbits. Asia-Pac. J. Sports Med. Arthrosc. Rehabil. Technol. 2014, 1, 54–61. [Google Scholar]
- Radisavljevic, A.; Stojanovic, D.B.; Perisic, S.; Djokic, V.; Radojevic, V.; Rajilic-Stojanovic, M.; Uskokovic, P.S. Cefazolin-loaded polycaprolactone fibers produced via different electrospinning methods: Characterization, drug release and antibacterial effect. Eur. J. Pharm. Sci. 2018, 124, 26–36. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, J.K.; Son, K.H.; Lee, J.W. PCL/sodium-alginate based 3D-printed dual drug delivery system with antibacterial activity for osteomyelitis therapy. Gels 2022, 8, 163. [Google Scholar] [CrossRef]
- Yazdi, I.K.; Murphy, M.B.; Loo, C.; Liu, X.; Ferrari, M.; Weiner, B.K.; Tasciotti, E. Cefazolin-loaded mesoporous silicon microparticles show sustained bactericidal effect against Staphylococcus aureus. J. Tissue Eng. 2014, 5, 2041731414536573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munir, M.U.; Ihsan, A.; Javed, I.; Ansari, M.T.; Bajwa, S.Z.; Bukhari, S.N.A.; Ahmed, A.; Malik, M.Z.; Khan, W.S. Controllably biodegradable hydroxyapatite nanostructures for cefazolin delivery against antibacterial aesistance. ACS Omega 2019, 4, 7524–7532. [Google Scholar] [CrossRef] [Green Version]
- Rath, G.; Hussain, T.; Chauhana, G.; Garg, T.; Goyall, A.K. Fabrication and characterization of cefazolin-loaded nanofibrous mats for the recovery of post-surgical wound. Artif. Cells Nanomed. Biotechnol. 2016, 44, 1783–1792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rath, G.; Hussain, T.; Chauhana, G.; Garg, T.; Goyall, A.K. Development and characterization of cefazolin loaded zinc oxide nanoparticles composite gelatin nanofiber mats for postoperative surgical wounds. Mater. Sci. Eng. C 2016, 58, 242–253. [Google Scholar] [CrossRef]
- Wang, H.; Dong, H.; Kang, C.G.; Lin, C.; Ye, X.; Zhao, Y.L. Preliminary exploration of the development of a collagenous artificial dura mater for sustained antibiotic release. Chin. Med. J. 2013, 126, 3329–3333. [Google Scholar] [PubMed]
- Kaplan, M.; Akgun, B.; Demirdag, K.; Akpolat, N.; Kozan, S.K.; Cagasar, O.; Yakar, H. Use of antibiotic-impregnated DuraGen ® to reduce the risk of infection in dura repair: An in vitro study. Cen. Eur. Neurosurg. 2011, 72, 75–77. [Google Scholar] [CrossRef]
- Suwanprateeb, J.; Thammarakcharoen, F.; Phanphiriya, P.; Chokevivat, W.; Suvannapruk, W.; Chernchujit, B. Preparation and characterizations of antibiotic impregnated microporous nano-hydroxyapatite for osteomyelitis treatment. Biomed. Eng. Appl. Basis Commun. 2014, 26, 1450041. [Google Scholar] [CrossRef]
- Guillen, G.R.; Pan, Y.; Li, M.; Hoek, E.M.V. Preparation and characterization of membranes formed by nonsolvent induced phase separation: A review. Ind. Eng. Chem. Res. 2011, 50, 3798–3817. [Google Scholar] [CrossRef]
- Tan, X.M.; Rodrigue, D. A review on porous polymeric membrane preparation. Part I: Production techniques with polysulfone and poly (vinylidene fluoride). Polymers 2019, 11, 1160. [Google Scholar] [CrossRef] [PubMed]
- van de Witte, P.; Dijkstra, P.J.; van den Berg, J.W.A.; Feijen, J. Phase separation processes in polymer solutions in relation to membrane formation. J. Membr. Sci. 1996, 117, 1–31. [Google Scholar] [CrossRef] [Green Version]
- Bashkatov, A.N.; Genina, E.A.; Sinichkin, Y.P.; Kochubey, V.I.; Lakodina, N.A.; Tuchin, V.V. Glucose and mannitol diffusion in human dura mater. Biophys. J. 2003, 85, 3310–3318. [Google Scholar] [CrossRef] [Green Version]
- Van Noort, R.; Black, M.M.; Martin, T.R.P.; Meanley, S. A study of the uniaxial mechanical properties of human dura mater preserved in glycerol. Biomaterials 1981, 2, 41–45. [Google Scholar] [CrossRef]
- Opálková Šišková, A.; Bucková, M.; Kroneková, Z.; Kleinová, A.; Nagy, Š.; Rydz, J.; Opálek, A.; Sláviková, M.; Eckstein Andicsová, A. The drug-loaded electrospun poly(ε-caprolactone) mats for therapeutic application. Nanomaterials 2021, 11, 922. [Google Scholar] [CrossRef]
- Gao, Y.; Li, J.; Xu, C.; Hou, Z.; Yang, L. Mechanical properties and drug loading rate of a polycaprolactone 5-fluorouracil controlled drug delivery system. Mater. Res. Express 2021, 8, 095302. [Google Scholar] [CrossRef]
- Glover, K.; Mathew, E.; Pitzanti, G.; Magee, E.; Lamprou, D. 3D bioprinted scaffolds for diabetic wound healing applications. Drug Deliv. Transl. Res. 2022. [Google Scholar] [CrossRef]
- Rychter, M.; Baranowska-Korczyc, A.; Milanowski, B.; Jarek, M.; Maciejewska, B.M.; Coy, E.L.; Lulek, J. Cilostazol-loaded poly(ε-caprolactone) electrospun drug delivery System for cardiovascular applications. Pharm. Res. 2018, 35, 32. [Google Scholar] [CrossRef] [Green Version]
- van Noort, R.; Martin, T.R.; Black, M.M.; Barker, A.T.; Montero, C.G. The mechanical properties of human dura mater and the effects of storage media. Clin. Phys. Physiol. Meas. 1981, 2, 197–203. [Google Scholar] [CrossRef]
- Zarzur, E. Mechanical properties of the human lumbar dura mater. Arq. Neuropsiquiatr. 1996, 54, 455–460. [Google Scholar] [CrossRef] [Green Version]
- Kamaraj, N.; Rajaguru, P.Y.; Issac, P.K.; Sundaresan, S. Fabrication, characterization, in vitro drug release and glucose uptake activity of 14-deoxy, 11, 12-didehydroandrographolide loaded polycaprolactone nanoparticles. Asian J. Pharm. Sci. 2017, 12, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.; Won, Y.Y. Phenomenology of the initial burst release of drugs from PLGA microparticles. ACS Biomater. Sci. Eng. 2020, 6, 6053–6062. [Google Scholar] [CrossRef]
- Hu, X.; Liu, S.; Zhou, G.; Huang, Y.; Xie, Z.; Jing, X. Electrospinning of polymeric nanofibers for drug delivery applications. J. Control. Release 2014, 185, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Karuppuswamy, P.; Reddy, V.J.; Navaneethan, B.; Luwang, L.A.; Ramakrishna, S. Polycaprolactone nanofibers for the controlled release of tetracycline hydrochloride. Mater. Lett. 2015, 141, 180–186. [Google Scholar] [CrossRef]
- Liu, D.Q.; Cheng, Z.Q.; Feng, Q.J.; Li, H.J.; Ye, S.F.; Teng, B. Polycaprolactone nanofibres loaded with 20(S)-protopanaxadiol for in vitro and in vivo anti-tumour activity study. R. Soc. Open Sci. 2018, 5, 180137. [Google Scholar] [CrossRef] [Green Version]
- Frantz, V.K. Absorbable Cotton, Paper and Gauze: (Oxidized Cellulose). Ann. Surg. 1943, 118, 116–126. [Google Scholar] [CrossRef]
- Spangler, D.; Rothenburger, S.; Nguyen, K.; Jampani, H.; Weiss, S.; Bhende, S. In Vitro Antimicrobial Activity of Oxidized Regenerated Cellulose against Antibiotic-Resistant Microorganisms. Surg. Infect. 2003, 4, 255–262. [Google Scholar] [CrossRef]
- Huang, X.; Brazel, C.S. On the importance and mechanisms of burst release in matrix-controlled drug delivery systems. J. Control. Release 2001, 73, 121–136. [Google Scholar] [CrossRef]
- Page, C.P.; Bohnen, J.M.; Fletcher, J.R.; McManus, A.T.; Solomkin, J.S.; Wittmann, D.H. Antimicrobial prophylaxis for surgical wounds. Guidelines for clinical care. Arch. Surg. 1993, 128, 79–88. [Google Scholar] [CrossRef]
- Patel, S.; Thompson, D.; Innocent, S.; Narbad, V.; Selway, R.; Barkas, K. Risk factors for surgical site infections in neurosurgery. Ann. R. Coll. Surg. Engl. 2019, 101, 220–225. [Google Scholar] [CrossRef]
- Mindermann, T. Empirically adapted or personalized antibiotic prophylaxis in select cranial neurosurgery? Acta Neurochir. 2021, 163, 365–367. [Google Scholar] [CrossRef] [PubMed]






| Samples | Cefazolin (g) | NMP (mL) | PCL (g) | ORC Impregnation |
|---|---|---|---|---|
| P20 | 0 | 100 | 20 | Yes |
| P20_25 | 2.5 | 100 | 20 | Yes |
| P20_50 | 5.0 | 100 | 20 | Yes |
| P20_75 | 7.5 | 100 | 20 | Yes |
| P20_100 | 10.0 | 100 | 20 | Yes |
| PCL | 0 | 100 | 20 | No |
| PCL_25 | 2.5 | 100 | 20 | No |
| PCL_50 | 5.0 | 100 | 20 | No |
| PCL_75 | 7.5 | 100 | 20 | No |
| PCL_100 | 10.0 | 100 | 20 | No |
| Samples | Total Cefazolin Content (mg Drug per 100 mg Sample) |
|---|---|
| P20_25 | 1.94 ± 0.15 |
| P20_50 | 2.77 ± 0.17 |
| P20_75 | 3.61 ± 0.07 |
| P20_100 | 5.54 ± 0.09 |
| PCL_25 | 1.74 ± 0.10 |
| PCL-50 | 2.49 ± 0.13 |
| PCL_75 | 2.80 ± 0.11 |
| PCL_100 | 3.28 ± 0.11 |
| Well Number | Cefazolin Concentration (μg/mL) | Bacterial Growth (х = No Growth; Clear Solution, ✓= Growth; Turbid Solution) |
|---|---|---|
| 1 | 5.0 | х |
| 2 | 2.5 | х |
| 3 | 1.50 | х |
| 4 | 0.650 | х |
| 5 | 0.3150 | х |
| 6 | 0.1563 | ✓ |
| 7 | 0.0780 | ✓ |
| 8 | 0.0390 | ✓ |
| 9 | 0.0195 | ✓ |
| 10 | 0.0 | ✓ |
| 11 | Nutrient broth (NB), no bacterial inoculum | х |
| 12 | NB + aCSF, no bacterial inoculum | х |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanpakitwattana, A.; Suvannapruk, W.; Chumnanvej, S.; Hemstapat, R.; Suwanprateeb, J. Cefazolin Loaded Oxidized Regenerated Cellulose/Polycaprolactone Bilayered Composite for Use as Potential Antibacterial Dural Substitute. Polymers 2022, 14, 4449. https://doi.org/10.3390/polym14204449
Sanpakitwattana A, Suvannapruk W, Chumnanvej S, Hemstapat R, Suwanprateeb J. Cefazolin Loaded Oxidized Regenerated Cellulose/Polycaprolactone Bilayered Composite for Use as Potential Antibacterial Dural Substitute. Polymers. 2022; 14(20):4449. https://doi.org/10.3390/polym14204449
Chicago/Turabian StyleSanpakitwattana, Arunnee, Waraporn Suvannapruk, Sorayouth Chumnanvej, Ruedee Hemstapat, and Jintamai Suwanprateeb. 2022. "Cefazolin Loaded Oxidized Regenerated Cellulose/Polycaprolactone Bilayered Composite for Use as Potential Antibacterial Dural Substitute" Polymers 14, no. 20: 4449. https://doi.org/10.3390/polym14204449