The Treatment of Keloid Scars via Modulating Heterogeneous Gelatin-Structured Composite Microneedles to Control Transdermal Dual-Drug Release
Abstract
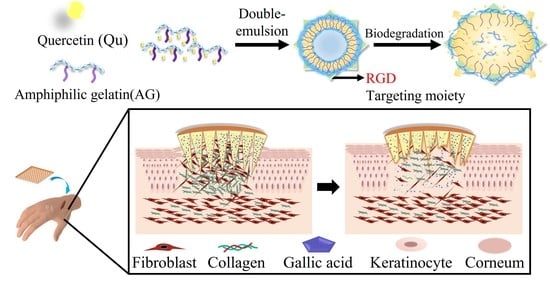
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Amphiphilic Gelatin
2.3. Preparation of Quercetin-Loaded Amphiphilic Gelatin Nanocarrier
2.4. Characterization of QAGNs
2.5. Encapsulation Efficiency and Loading Capacity
2.6. Qu Release Study
2.7. Synthesis of MN Arrays Patch
2.8. Mechanical Test of MN Patch
2.9. In Vitro Penetration Capability of MN Patch
2.10. In Vitro Drug Permeation Study
2.11. Cell Proliferation and Viability Assay of L929 Fibroblasts
2.12. Aniline Blue Staining
2.13. Measurement of Reactive Oxygen Species Generation
2.14. Real-Time Quantitative Polymerase Chain Reaction
2.15. Statistical Analysis
3. Results
3.1. Preparation and Characterization of QAGNs
3.2. Preparation and Characterization of MNs
3.3. MN Functionality Testing
3.4. In Vitro Drug Release Profile of Dual-Drug-Loaded MNs
3.5. Cell Proliferation and Viability Assay of L929 Fibroblasts
3.6. Aniline Blue Staining of L929 Fibroblasts
3.7. qRT-PCR
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, S.; Wang, Q.; Huang, A.; Fan, H.; Yan, S.; Zhang, Q. Advances in Skin Wound and Scar Repair by Polymer Scaffolds. Molecules 2021, 26, 6110. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; DiPietro, L.A. Factors Affecting Wound Healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Müller, W.E.G.; Relkovic, D.; Ackermann, M.; Wang, S.; Neufurth, M.; Radicevic, A.P.; Ushijima, H.; Schröder, H.C.; Wang, X. Enhancement of Wound Healing in Normal and Diabetic Mice by Topical Application of Amorphous Polyphosphate. Superior Effect of a Host-Guest Composite Material Composed of Collagen (Host) and Polyphosphate (Guest). Polymers 2017, 9, 300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gauglitz, G.G.; Korting, H.C.; Pavicic, T.; Ruzicka, T.; Jeschke, M.G. Hypertrophic Scarring and Keloids: Pathomechanisms and Current and Emerging Treatment Strategies. Mol. Med. 2011, 17, 113–125. [Google Scholar] [CrossRef]
- Limandjaja, G.C.; Niessen, F.B.; Scheper, R.J.; Gibbs, S. Hypertrophic scars and keloids: Overview of the evidence and practical guide for differentiating between these abnormal scars. Exp. Dermatol. 2021, 30, 146–161. [Google Scholar] [CrossRef]
- Eishi, K.; Bae, S.; Ogawa, F.; Hamasaki, Y.; Shimizu, K.; Katayama, I. Silicone gel sheets relieve pain and pruritus with clinical improvement of keloid: Possible target of mast cells. J. Dermatol. Treat. 2003, 14, 248–252. [Google Scholar] [CrossRef]
- Comino-Sanz, I.M.; López-Franco, M.D.; Castro, B.; Pancorbo-Hidalgo, P.L. The Role of Antioxidants on Wound Healing: A Review of the Current Evidence. J. Clin. Med. 2021, 10, 3558. [Google Scholar] [CrossRef]
- Beken, B.; Serttas, R.; Yazicioglu, M.; Turkekul, K.; Erdogan, S. Quercetin Improves Inflammation, Oxidative Stress, and Impaired Wound Healing in Atopic Dermatitis Model of Human Keratinocytes. Pediatr. Allergy Immunol. Pulmonol. 2020, 33, 69–79. [Google Scholar] [CrossRef]
- Kahkeshani, N.; Farzaei, F.; Fotouhi, M.; Alavi, S.S.; Bahramsoltani, R.; Naseri, R.; Momtaz, S.; Abbasabadi, Z.; Rahimi, R.; Farzaei, M.H.; et al. Pharmacological effects of gallic acid in health and disease: A mechanistic review. Iran. J. Basic Med. Sci. 2019, 22, 225–237. [Google Scholar] [CrossRef]
- Wang, X.; Liu, K.; Ruan, M.; Yang, J.; Gao, Z. Gallic acid inhibits fibroblast growth and migration in keloids through the AKT/ERK signaling pathway. Acta Biochim. Biophys. Sin. 2018, 50, 1114–1120. [Google Scholar] [CrossRef]
- Zamudio-Cuevas, Y.; Andonegui-Elguera, M.A.; Aparicio-Juárez, A.; Aguillón-Solís, E.; Martínez-Flores, K.; Ruvalcaba-Paredes, E.; Velasquillo-Martínez, C.; Ibarra, C.; Martínez-López, V.; Gutiérrez, M.; et al. The enzymatic poly(gallic acid) reduces pro-inflammatory cytokines in vitro, a potential application in inflammatory diseases. Inflammation 2021, 44, 174–185. [Google Scholar] [CrossRef] [PubMed]
- Kant, V.; Jangir, B.L.; Sharma, M.; Kumar, V.; Joshi, V.G. Topical application of quercetin improves wound repair and regeneration in diabetic rats. Immunopharmacol. Immunotoxicol. 2021, 43, 536–553. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wang, X.-F.; Wang, Z.-C.; Lou, D.; Fang, Q.-Q.; Hu, Y.-Y.; Zhao, W.-Y.; Zhang, L.-Y.; Wu, L.-H.; Tan, W.-Q. Current potential therapeutic strategies targeting the TGF-β/Smad signaling pathway to attenuate keloid and hypertrophic scar formation. Biomed. Pharmacother. 2020, 129, 110287. [Google Scholar] [CrossRef] [PubMed]
- Shirakami, E.; Yamakawa, S.; Hayashida, K. Strategies to prevent hypertrophic scar formation: A review of therapeutic interventions based on molecular evidence. Burn. Trauma 2020, 8, tkz003. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y.; Chen, H.; Sun, Y.; Fan, Z.; Cheng, H. Quercetin inhibits fibroblasts proliferation and reduces surgery-induced epidural fibrosis via the autophagy-mediated PI3K/Akt/mTOR pathway. Bioengineered 2022, 13, 9973–9986. [Google Scholar] [CrossRef]
- Wadhwa, K.; Kadian, V.; Puri, V.; Bhardwaj, B.Y.; Sharma, A.; Pahwa, R.; Rao, R.; Gupta, M.; Singh, I. New insights into quercetin nanoformulations for topical delivery. Phytomed. Plus 2022, 2, 100257. [Google Scholar] [CrossRef]
- Roberts, M.; Mohammed, Y.; Pastore, M.; Namjoshi, S.; Yousef, S.; Alinaghi, A.; Haridass, I.; Abd, E.; Leite-Silva, V.; Benson, H.; et al. Topical and cutaneous delivery using nanosystems. J. Control. Release 2017, 247, 86–105. [Google Scholar] [CrossRef] [Green Version]
- Lim, D.-J.; Kim, H.-J. Microneedles in Action: Microneedling and Microneedles-Assisted Transdermal Delivery. Polymers 2022, 14, 1608. [Google Scholar] [CrossRef]
- Jiang, X.; Zhao, H.; Li, W. Microneedle-Mediated Transdermal Delivery of Drug-Carrying Nanoparticles. Front. Bioeng. Biotechnol. 2022, 10, 840395. [Google Scholar] [CrossRef]
- Su, L.-C.; Chen, M.-C. Efficient delivery of nanoparticles to deep skin layers using dissolvable microneedles with an extended-length design. J. Mater. Chem. B 2017, 5, 3355–3363. [Google Scholar] [CrossRef]
- Rzhevskiy, A.S.; Singh, T.R.R.; Donnelly, R.F.; Anissimov, Y.G. Microneedles as the technique of drug delivery enhancement in diverse organs and tissues. J. Control. Release 2018, 270, 184–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chenthamara, D.; Subramaniam, S.; Ramakrishnan, S.G.; Krishnaswamy, S.; Essa, M.M.; Lin, F.H.; Qoronfleh, M.W. Therapeutic efficacy of nanoparticles and routes of administration. Biomater. Res. 2019, 23, 20. [Google Scholar] [CrossRef]
- Simon, L.; Vincent, M.; Le Saux, S.; Lapinte, V.; Marcotte, N.; Morille, M.; Dorandeu, C.; Devoisselle, J.; Bégu, S. Polyoxazolines based mixed micelles as PEG free formulations for an effective quercetin antioxidant topical delivery. Int. J. Pharm. 2019, 570, 118516. [Google Scholar] [CrossRef]
- Hatahet, T.; Morille, M.; Hommoss, A.; Devoisselle, J.-M.; Müller, R.; Bégu, S. Liposomes, lipid nanocapsules and smartCrystals®: A comparative study for an effective quercetin delivery to the skin. Int. J. Pharm. 2018, 542, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.-S.; Chen, J.-Y.; Chiang, M.-Y.; Hou, K.-T.; Li, W.-M.; Chang, S.-J.; Chen, S.-Y. Using the interplay of magnetic guidance and controlled TGF-β release from protein-based nanocapsules to stimulate chondrogenesis. Int. J. Nanomed. 2018, 13, 3177–3188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, S.-W.; Chen, Y.-J.; Chen, C.-J.; Liu, J.-T.; Yang, C.-Y.; Tsai, J.-H.; Lu, H.-E.; Chen, S.-Y.; Chang, S.-J. High-Density Horizontal Stacking of Chondrocytes via the Synergy of Biocompatible Magnetic Gelatin Nanocarriers and Internal Magnetic Navigation for Enhancing Cartilage Repair. Polymers 2022, 14, 809. [Google Scholar] [CrossRef]
- Yang, S.-W.; Ku, K.-C.; Chen, S.-Y.; Kuo, S.-M.; Chen, I.-F.; Wang, T.-Y.; Chang, S.-J. Development of chondrocyte-seeded electrosprayed nanoparticles for repair of articular cartilage defects in rabbits. J. Biomater. Appl. 2018, 32, 800–812. [Google Scholar] [CrossRef]
- Larrañeta, E.; Moore, J.; Vicente-Pérez, E.M.; González-Vázquez, P.; Lutton, R.; Woolfson, A.D.; Donnelly, R.F. A proposed model membrane and test method for microneedle insertion studies. Int. J. Pharm. 2014, 472, 65–73. [Google Scholar] [CrossRef] [Green Version]
- Larrañeta, E.; Stewart, S.; Fallows, S.J.; Birkhäuer, L.L.; McCrudden, M.T.; Woolfson, A.D.; Donnelly, R.F. A facile system to evaluate in vitro drug release from dissolving microneedle arrays. Int. J. Pharm. 2016, 497, 62–69. [Google Scholar] [CrossRef] [Green Version]
- Paudel, K.S.; Milewski, M.; Swadley, C.L.; Brogden, N.K.; Ghosh, P.; Stinchcomb, A.L. Challenges and opportunities in dermal/transdermal delivery. Ther. Deliv. 2010, 1, 109–131. [Google Scholar] [CrossRef]
- Gittard, S.D.; Chen, B.; Xu, H.; Ovsianikov, A.; Chichkov, B.; Monteiro-Riviere, N.; Narayan, R.J. The effects of geometry on skin penetration and failure of polymer microneedles. J. Adhes. Sci. Technol. 2013, 27, 227–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phan, T.-T.; Sun, L.; Bay, B.-H.; Chan, S.-Y.; Lee, S.-T. Dietary Compounds Inhibit Proliferation and Contraction of Keloid and Hypertrophic Scar-Derived Fibroblasts In Vitro: Therapeutic Implication for Excessive Scarring. J. Trauma 2003, 54, 1212–1224. [Google Scholar] [CrossRef] [PubMed]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Friedman, D.W.; Boyd, C.D.; MacKenzie, J.W.; Norton, P.; Olson, R.M.; Deak, S.B. Regulation of Collagen Gene Expression in Keloids and Hypertrophic Scars. J. Surg. Res. 1993, 55, 214–222. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, Q.; Mo, W.; Feng, J.; Li, S.; Li, J.; Liu, T.; Xu, S.; Wang, W.; Lu, X.; et al. Quercetin prevents hepatic fibrosis by inhibiting hepatic stellate cell activation and reducing autophagy via the TGF-β1/Smads and PI3K/Akt pathways. Sci. Rep. 2017, 7, 9289. [Google Scholar] [CrossRef]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-J.; Cheng, H.-W.; Yen, W.-Y.; Tsai, J.-H.; Yeh, C.-Y.; Chen, C.-J.; Liu, J.T.; Chen, S.-Y.; Chang, S.-J. The Treatment of Keloid Scars via Modulating Heterogeneous Gelatin-Structured Composite Microneedles to Control Transdermal Dual-Drug Release. Polymers 2022, 14, 4436. https://doi.org/10.3390/polym14204436
Chen Y-J, Cheng H-W, Yen W-Y, Tsai J-H, Yeh C-Y, Chen C-J, Liu JT, Chen S-Y, Chang S-J. The Treatment of Keloid Scars via Modulating Heterogeneous Gelatin-Structured Composite Microneedles to Control Transdermal Dual-Drug Release. Polymers. 2022; 14(20):4436. https://doi.org/10.3390/polym14204436
Chicago/Turabian StyleChen, Yong-Ji, Hung-Wei Cheng, Wan-Yu Yen, Jen-Hao Tsai, Chin-Yi Yeh, Ching-Jung Chen, Jen Tsai Liu, San-Yuan Chen, and Shwu-Jen Chang. 2022. "The Treatment of Keloid Scars via Modulating Heterogeneous Gelatin-Structured Composite Microneedles to Control Transdermal Dual-Drug Release" Polymers 14, no. 20: 4436. https://doi.org/10.3390/polym14204436