Amphiphilic Diblock Copolymers Bearing Poly(Ethylene Glycol) Block: Hydrodynamic Properties in Organic Solvents and Water Micellar Dispersions, Effect of Hydrophobic Block Chemistry on Dispersion Stability and Cytotoxicity
Abstract
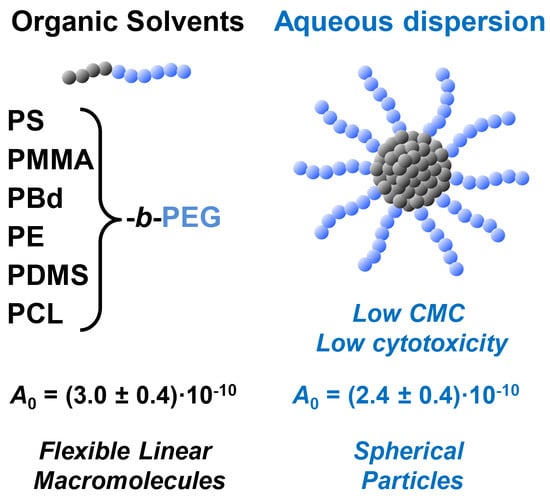
:1. Introduction
2. Materials and Methods
2.1. General Comments
2.2. Preparation of Block Copolymer Micelles
2.3. Gel Permeation Chromatography (GPC)
2.4. Dynamic Light Scattering (DLS)
2.5. Analytical Ultracentrifugation (AUC)
2.6. Viscometry
2.7. Cell Culture and Cell Viability (MTT) Assay
3. Results and Discussion
3.1. Block Copolymers Used in the Study
- Glass transition (Tg) temperature; this parameter governs the ability of micelles to equilibrate; the micelles with “glassy” cores (i.e., composed of blocks with Tg > room temperature, RT) are assumed to be irreversible (“frozen”) ones and vice versa [2]. In turn, ‘frozen’ micelles are much more stable towards any rearrangements upon variations of ambient conditions and disintegration upon dilution, and this feature is quite beneficial in numerous applications. In the set of polymers we used, PS-b-PEG and PMMA-b-PEG (with Tg are of ca. 100 °C in bulk [41]) are expected to form “frozen” micelles while PDMS-b-PEG, PE-b-PEG, and PBd-b-PEG (bulk Tg for PDMS, PE, and PBd are of ca. −120 °C, −80… −120 °C, and −60… −100 °C, respectively [41]), are expected to display much higher chain mobility inside cores; in the case of PCL-b-PEG, the core structure is more complex, since bulk PCL is a semicrystalline polymer with low Tg of −60 °C, but a high melting point of 60 °C [42];
- Hydrophobicity; the higher hydrophobicity, the lower critical micelle concentration (CMC); additionally, micellar cores composed of highly hydrophobic blocks (such as PS) were reported to be almost free of water. In our set, most block copolymers are strongly hydrophobic; nevertheless, PCL and PMMA blocks contain relatively polar ester groups and can potentially be plasticized by water to some extent;
- Gas permeability; this requirement is not general and relates to our recent study where we have outlined the prospects of polymer micelles application in intracellular lifetime oxygen biosensing [39,43]: in this particular aspect, block copolymer micelles serve as nanocontainers for phosphorescent organometallic complexes that rapidly and reversibly respond to the changes in oxygen concentration by varying their luminescence lifetime. We have shown that, in the micelles, the hydrophobic phosphors are embedded into micellar cores, where the outer shell strongly protects the reporter molecule from interactions with biomolecules, thereby preserving its lifetime response from various biasing factors [39]. Obviously, the highest oxygen sensing response can be anticipated in the case of high gas permeability of the material comprising the core; in this context, we added PDMS-b-PEG to the set of block copolymers since PDMS has almost 2–3 orders of magnitude higher oxygen permeability [44] compared to other block copolymers of the series.
3.2. Hydrodynamic and Molecular Characteristics of Block Copolymers in Organic Solvents
3.3. Preparation and Stability of Block Copolymer Micelles in Aqueous Dispersion
3.4. Hydrodynamic Behavior of Block Copolymer Micelles in Aqueous Dispersion
3.5. Cytotoxicity Study of Block Copolymer Micelles in Aqueous Dispersion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Riess, G. Micellization of Block Copolymers. Prog. Polym. Sci. 2003, 28, 1107–1170. [Google Scholar] [CrossRef] [Green Version]
- Gohy, J.F. Block Copolymer Micelles. Adv. Polym. Sci. 2005, 190, 65–136. [Google Scholar] [CrossRef]
- Cabral, H.; Miyata, K.; Osada, K.; Kataoka, K. Block Copolymer Micelles in Nanomedicine Applications. Chem. Rev. 2018, 118, 6844–6892. [Google Scholar] [CrossRef] [Green Version]
- Hwang, D.; Ramsey, J.D.; Kabanov, A.V. Polymeric Micelles for the Delivery of Poorly Soluble Drugs: From Nanoformulation to Clinical Approval. Adv. Drug Deliv. Rev. 2020, 156, 80–118. [Google Scholar] [CrossRef] [PubMed]
- Borisov, S.M.; Mayr, T.; Klimant, I. Poly(Styrene-Block-Vinylpyrrolidone) Beads as a Versatile Material for Simple Fabrication of Optical Nanosensors. Anal. Chem. 2008, 80, 573–582. [Google Scholar] [CrossRef] [PubMed]
- Borisov, S.M.; Nuss, G.; Klimant, I. Red Light-Excitable Oxygen Sensing Materials Based on Platinum(II) and Palladium(II) Benzoporphyrins. Anal. Chem. 2008, 80, 9435–9442. [Google Scholar] [CrossRef]
- Borisov, S.M.; Klimant, I. Luminescent Nanobeads for Optical Sensing and Imaging of Dissolved Oxygen. Microchim. Acta 2009, 164, 7–15. [Google Scholar] [CrossRef]
- Ehgartner, J.; Strobl, M.; Bolivar, J.M.; Rabl, D.; Rothbauer, M.; Ertl, P.; Borisov, S.M.; Mayr, T. Simultaneous Determination of Oxygen and PH Inside Microfluidic Devices Using Core–Shell Nanosensors. Anal. Chem. 2016, 88, 9796–9804. [Google Scholar] [CrossRef]
- Wang, X.; Stolwijk, J.A.; Lang, T.; Sperber, M.; Meier, R.J.; Wegener, J.; Wolfbeis, O.S. Ultra-Small, Highly Stable, and Sensitive Dual Nanosensors for Imaging Intracellular Oxygen and PH in Cytosol. J. Am. Chem. Soc. 2012, 134, 17011–17014. [Google Scholar] [CrossRef] [PubMed]
- Su, F.; Alam, R.; Mei, Q.; Tian, Y.; Youngbull, C.; Johnson, R.H.; Meldrum, D.R. Nanostructured Oxygen Sensor—Using Micelles to Incorporate a Hydrophobic Platinum Porphyrin. PLoS ONE 2012, 7, e33390. [Google Scholar] [CrossRef] [PubMed]
- Qu, P.; Kuepfert, M.; Ahmed, E.; Liu, F.; Weck, M. Cross-Linked Polymeric Micelles as Catalytic Nanoreactors. Eur. J. Inorg. Chem. 2021, 2021, 1420–1427. [Google Scholar] [CrossRef]
- Bronstein, L.; Krämer, E.; Berton, B.; Burger, C.; Förster, S.; Antonietti, M. Successive Use of Amphiphilic Block Copolymers as Nanoreactors and Templates: Preparation of Porous Silica with Metal Nanoparticles. Chem. Mater. 1999, 11, 1402–1405. [Google Scholar] [CrossRef]
- Bronstein, L.M.; Chernyshov, D.M.; Timofeeva, G.I.; Dubrovina, L.V.; Valetsky, P.M.; Obolonkova, E.S.; Khokhlov, A.R. Interaction of Polystyrene- Block -Poly(Ethylene Oxide) Micelles with Cationic Surfactant in Aqueous Solutions. Metal Colloid Formation in Hybrid Systems. Langmuir 2000, 16, 3626–3632. [Google Scholar] [CrossRef]
- Swisher, J.H.; Jibril, L.; Petrosko, S.H.; Mirkin, C.A. Nanoreactors for Particle Synthesis. Nat. Rev. Mater. 2022, 7, 428–448. [Google Scholar] [CrossRef]
- Xu, R.; Winnik, M.A.; Hallett, F.R.; Riess, G.; Croucher, M.D. Light-Scattering Study of the Association Behavior of Styrene-Ethylene Oxide Block Copolymers in Aqueous Solution. Macromolecules 1991, 24, 87–93. [Google Scholar] [CrossRef]
- Xu, R.; Winnik, M.A.; Riess, G.; Chu, B.; Croucher, M.D. Micellization of Polystyrene-Poly(Ethylene Oxide) Block Copolymers in Water. 5. A Test of the Star and Mean-Field Models. Macromolecules 1992, 25, 644–652. [Google Scholar] [CrossRef]
- Bronstein, L.M.; Chernyshov, D.M.; Timofeeva, G.I.; Dubrovina, L.V.; Valetsky, P.M.; Khokhlov, A.R. Polystyrene-Block-Poly(Ethylene Oxide) Micelles in Aqueous Solution. Langmuir 1999, 15, 6195–6200. [Google Scholar] [CrossRef]
- Jada, A.; Hurtrez, G.; Siffert, B.; Riess, G. Structure of Polystyrene-Block-Poly(Ethylene Oxide) Diblock Copolymer Micelles in Water. Macromol. Chem. Phys. 1996, 197, 3697–3710. [Google Scholar] [CrossRef]
- Atanase, L.I.; Lerch, J.-P.; Caprarescu, S.; Iurciuc Tincu, C.E.; Riess, G. Micellization of PH-Sensitive Poly(Butadiene)- Block -Poly(2 Vinylpyridine)- Block -Poly(Ethylene Oxide) Triblock Copolymers: Complex Formation with Anionic Surfactants. J. Appl. Polym. Sci. 2017, 134, 45313. [Google Scholar] [CrossRef]
- Hickl, P.; Ballauff, M.; Jada, A. Small-Angle X-Ray Contrast-Variation Study of Micelles Formed by Poly(Styrene)-Poly(Ethylene Oxide) Block Copolymers in Aqueous Solution. Macromolecules 1996, 29, 4006–4014. [Google Scholar] [CrossRef]
- Mortensen, K.; Brown, W.; Almdal, K.; Alami, E.; Jada, A. Structure of PS−PEO Diblock Copolymers in Solution and the Bulk State Probed Using Dynamic Light-Scattering and Small-Angle Neutron-Scattering and Dynamic Mechanical Measurements. Langmuir 1997, 13, 3635–3645. [Google Scholar] [CrossRef]
- Khan, T.N.; Mobbs, R.H.; Price, C.; Quintana, J.R.; Stubbersfield, R.B. Synthesis and Colloidal Behaviour of a Polystyrene-b-Poly(Ethylene Oxide) Block Copolymer. Eur. Polym. J. 1987, 23, 191–194. [Google Scholar] [CrossRef]
- Yu, K.; Eisenberg, A. Multiple Morphologies in Aqueous Solutions of Aggregates of Polystyrene- Block -Poly(Ethylene Oxide) Diblock Copolymers. Macromolecules 1996, 29, 6359–6361. [Google Scholar] [CrossRef]
- Zhao, C.L.; Winnik, M.A.; Riess, G.; Croucher, M.D. Fluorescence Probe Techniques Used to Study Micelle Formation in Water-Soluble Block Copolymers. Langmuir 1990, 6, 514–516. [Google Scholar] [CrossRef]
- Wilhelm, M.; Zhao, C.L.; Wang, Y.; Xu, R.; Winnik, M.A.; Mura, J.L.; Riess, G.; Croucher, M.D. Poly(Styrene-Ethylene Oxide) Block Copolymer Micelle Formation in Water: A Fluorescence Probe Study. Macromolecules 1991, 24, 1033–1040. [Google Scholar] [CrossRef]
- Luo, L.; Tam, J.; Maysinger, D.; Eisenberg, A. Cellular Internalization of Poly(Ethylene Oxide)-b-Poly(ε-Caprolactone) Diblock Copolymer Micelles. Bioconjug. Chem. 2002, 13, 1259–1265. [Google Scholar] [CrossRef]
- Mahmud, A.; Lavasanifar, A. The Effect of Block Copolymer Structure on the Internalization of Polymeric Micelles by Human Breast Cancer Cells. Colloids Surf. B Biointerfaces 2005, 45, 82–89. [Google Scholar] [CrossRef]
- Carstens, M.G.; van Nostrum, C.F.; Verrijk, R.; de Leede, L.G.J.; Crommelin, D.J.A.; Hennink, W.E. A Mechanistic Study on the Chemical and Enzymatic Degradation of PEG-Oligo(Ε-caprolactone) Micelles. J. Pharm. Sci. 2008, 97, 506–518. [Google Scholar] [CrossRef]
- Letchford, K.; Liggins, R.; Burt, H. Solubilization of Hydrophobic Drugs by Methoxy Poly(Ethylene Glycol)-Block-Polycaprolactone Diblock Copolymer Micelles: Theoretical and Experimental Data and Correlations. J. Pharm. Sci. 2008, 97, 1179–1190. [Google Scholar] [CrossRef]
- Wei, X.; Gong, C.; Gou, M.; Fu, S.; Guo, Q.; Shi, S.; Luo, F.; Guo, G.; Qiu, L.; Qian, Z. Biodegradable Poly(ɛ-Caprolactone)–Poly(Ethylene Glycol) Copolymers as Drug Delivery System. Int. J. Pharm. 2009, 381, 1–18. [Google Scholar] [CrossRef]
- Grossen, P.; Witzigmann, D.; Sieber, S.; Huwyler, J. PEG-PCL-Based Nanomedicines: A Biodegradable Drug Delivery System and Its Application. J. Control. Release 2017, 260, 46–60. [Google Scholar] [CrossRef] [PubMed]
- Desai, H.; Varade, D.; Aswal, V.K.; Goyal, P.S.; Bahadur, P. Micellar Characteristics of Diblock Polyacrylate–Polyethylene Oxide Copolymers in Aqueous Media. Eur. Polym. J. 2006, 42, 593–601. [Google Scholar] [CrossRef]
- Schuck, P. Size-Distribution Analysis of Macromolecules by Sedimentation Velocity Ultracentrifugation and Lamm Equation Modeling. Biophys. J. 2000, 78, 1606–1619. [Google Scholar] [CrossRef] [Green Version]
- Mächtle, W.; Börger, L. Analytical Ultracentrifugation of Polymers and Nanoparticles; Springer: New York, NY, USA, 2006; ISBN 3-540-23432-2. [Google Scholar]
- Perevyazko, I.; Gubarev, A.S.; Pavlov, G.M. Analytical Ultracentrifugation and Combined Molecular Hydrodynamic Approaches for Polymer Characterization. In Molecular Characterization of Polymers; Elsevier: Amsterdam, The Netherlands, 2021; pp. 223–259. [Google Scholar]
- COLLINS, K. Ions from the Hofmeister Series and Osmolytes: Effects on Proteins in Solution and in the Crystallization Process. Methods 2004, 34, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Huggins, M.L. The Viscosity of Dilute Solutions of Long-Chain Molecules. IV. Dependence on Concentration. J. Am. Chem. Soc. 1942, 64, 2716–2718. [Google Scholar] [CrossRef]
- Pamies, R.; Hernández Cifre, J.G.; del Carmen López Martínez, M.; García de la Torre, J. Determination of Intrinsic Viscosities of Macromolecules and Nanoparticles. Comparison of Single-Point and Dilution Procedures. Colloid Polym. Sci. 2008, 286, 1223–1231. [Google Scholar] [CrossRef]
- Elistratova, A.A.; Kritchenkov, I.S.; Lezov, A.A.; Gubarev, A.S.; Solomatina, A.I.; Kachkin, D.V.; Shcherbina, N.A.; Liao, Y.-C.; Liu, Y.-C.; Yang, Y.-Y.; et al. Lifetime Oxygen Sensors Based on Block Copolymer Micelles and Non-Covalent Human Serum Albumin Adducts Bearing Phosphorescent Near-Infrared Iridium(III) Complex. Eur. Polym. J. 2021, 159, 110761. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Brandrup, J.; Immergut, E.H.; Grulk, E.A. Polymer Handbook Fourth Edition; A Wiley-Interscience Publication: Toronto, ON, Canada, 1999. [Google Scholar]
- English, J.; Perrin, D. Polycaprolactone. In Handbook of Biodegradable Polymers; CRC Press: Boca Raton, FL, USA, 1998; pp. 63–77. [Google Scholar]
- Zharskaia, N.A.; Solomatina, A.I.; Liao, Y.-C.; Galenko, E.E.; Khlebnikov, A.F.; Chou, P.-T.; Chelushkin, P.S.; Tunik, S.P. Aggregation-Induced Ignition of Near-Infrared Phosphorescence of Non-Symmetric [Pt(C^N*N’^C’)] Complex in Poly(Caprolactone)-Based Block Copolymer Micelles: Evaluating the Alternative Design of Near-Infrared Oxygen Biosensors. Biosensors 2022, 12, 695. [Google Scholar] [CrossRef]
- Wang, X.; Wolfbeis, O.S. Optical Methods for Sensing and Imaging Oxygen: Materials, Spectroscopies and Applications. Chem. Soc. Rev. 2014, 43, 3666–3761. [Google Scholar] [CrossRef]
- Scott, D.J.; Harding, S.E.; Rowe, A.J. Diffusion-Deconvoluted Sedimentation Coefficient Distributions for the Analysis of Interacting and Non-Interacting Protein Mixtures. In Analytical Ultracentrifugation: Techniques and Methods; Royal Society of Chemistry: Cambridge, UK, 2005; pp. 26–50. [Google Scholar]
- Todd, G.P.; Haschemeyer, R.H. General Solution to the Inverse Problem of the Differential Equation of the Ultracentrifuge. Proc. Natl. Acad. Sci. USA 1981, 78, 6739–6743. [Google Scholar] [CrossRef] [Green Version]
- Isoglu, I.; Ozsoy, Y.; Isoglu, S. Advances in Micelle-Based Drug Delivery: Cross-Linked Systems. Curr. Top. Med. Chem. 2017, 17, 1469–1489. [Google Scholar] [CrossRef]
- Güven, O. Size Exclusion Chromatography of Poly(Ethylene Glycol). Br. Polym. J. 1986, 18, 391–393. [Google Scholar] [CrossRef]
- Tsvetkov, V.N.; Lavrenko, P.N.; Bushin, S.V. Hydrodynamic Invariant of Polymer Molecules. J. Polym. Sci. Polym. Chem. Ed. 1984, 22, 3447–3486. [Google Scholar] [CrossRef]
- Gou, M.; Men, K.; Shi, H.; Xiang, M.; Zhang, J.; Song, J.; Long, J.; Wan, Y.; Luo, F.; Zhao, X.; et al. Curcumin-Loaded Biodegradable Polymeric Micelles for Colon Cancer Therapy in Vitro and in Vivo. Nanoscale 2011, 3, 1558–1567. [Google Scholar] [CrossRef]
- Schramm, O.G.; Pavlov, G.M.; van Erp, H.P.; Meier, M.A.R.; Hoogenboom, R.; Schubert, U.S. A Versatile Approach to Unimolecular Water-Soluble Carriers: ATRP of PEGMA with Hydrophobic Star-Shaped Polymeric Core Molecules as an Alternative for PEGylation. Macromolecules 2009, 42, 1808–1816. [Google Scholar] [CrossRef]
- Lezov, A.; Gubarev, A.; Kaiser, T.; Tobaschus, W.; Tsvetkov, N.; Nischang, I.; Schubert, U.S.; Frey, H.; Perevyazko, I. “Hard” Sphere Behavior of “Soft”, Globular-like, Hyperbranched Polyglycerols—Extensive Molecular Hydrodynamic and Light Scattering Studies. Macromolecules 2020, 53, 9220–9233. [Google Scholar] [CrossRef]
- Gubarev, A.S.; Lezov, A.A.; Senchukova, A.S.; Vlasov, P.S.; Serkova, E.S.; Kuchkina, N.V.; Shifrina, Z.B.; Tsvetkov, N.V. Diels–Alder Hyperbranched Pyridylphenylene Polymer Fractions as Alternatives to Dendrimers. Macromolecules 2019, 52, 1882–1891. [Google Scholar] [CrossRef]
- Perevyazko, I.; Seiwert, J.; Schömer, M.; Frey, H.; Schubert, U.S.; Pavlov, G.M. Hyperbranched Poly(Ethylene Glycol) Copolymers: Absolute Values of the Molar Mass, Properties in Dilute Solution, and Hydrodynamic Homology. Macromolecules 2015, 48, 5887–5898. [Google Scholar] [CrossRef]
- Halperin, A. Polymeric Micelles: A Star Model. Macromolecules 1987, 20, 2943–2946. [Google Scholar] [CrossRef]




| Block Copolymer | N(PEG)/N(block) Expected a | N(PEG)/N(block) Experimental b | Mw, [g/mol] Calculated c | Đ Provided c | Mw, [g/mol] Experimental d | Đ Experi- mental d | <MsD>g, [g/mol] Absolute Values |
|---|---|---|---|---|---|---|---|
| PS35-b-PEG115 | 3.11:1.00 | 3.19:1.00 | 9300 | 1.06 | 11,500 | 1.04 | 11,800 |
| PMMA55-b-PEG95 | 1.77:1.00 | 1.57:1.00 | 11,000 | 1.15 | 9000 | 1.22 | 13,700 |
| PBd90-b-PEG130 | 1.48:1.00 | 1.40:1.00 | 11,000 | 1.04 | 15,800 | 1.05 | 9600 |
| PE40-b-PEG85 | 2.20:1.00 | n.d. e | 5400 | 1.11 | 6000 f | 1.28 f | 4200 |
| PDMS15-b-PEG115 | 8.42:1.00 | 8.36:1.00 | 6600 | 1.10 | 8000 | 1.07 | 5700 |
| PCL45-b-PEG115 | 2.59:1.00 | 4.51:1.00 | 11,800 | 1.18 | 14,600 | 1.15 | 9000 |
| Block Copolymer | [η], [cm3/g] | kH/−kK | <D0>107, [cm2/s] a | Rh, [nm] a | s0, [S] b | (f/fsph)0 b | MsD × 10−3, [g/mol] | A0 × 1010 |
|---|---|---|---|---|---|---|---|---|
| PS-b-PEG | 18.3 | 0.30/0.14 | 14.6 | 2.9 | 1.21 c | 1.8 | 11.8 | 3.22 |
| PMMA-b-PEG | 15.8 | 0.29/0.15 | 14.2 | 3.0 | 1.91 c | 1.6 | 13.7 | 3.14 |
| PBd-b-PEG | 24.9 | 0.43/0.12 | 12.9 | 3.3 | 0.70 c | 2.2 | 9.6 | 2.93 |
| PE-b-PEG | 13.7 | 0.45/0.09 | 22.5 | 1.9 | 0.59 | 1.6 | 4.2 | 3.2 |
| PDMS-b-PEG | 14.9 | 0.07/0.32 | 16.0 | 2.7 | 0.88 | 2.2 | 5.7 | 2.6 |
| PCL-b-PEG | 24.1 | 0.33/0.15 | 15.4 | 2.8 | 1.11 | 1.9 | 9.0 | 3.41 |
| Block Copolymer | DMSO | DMF | Alternative Solvent | CMCapp, [mg/L] |
|---|---|---|---|---|
| PS-b-PEG | − | + | n.i. a | 2.0 ± 1.3 |
| PMMA-b-PEG | − | − | + (THF) | 1.1 ± 0.5 |
| PBd-b-PEG | − | + | n.i. a | 14 ± 8 |
| PE-b-PEG | − | − | + (1,4-dioxane) | 11 ± 5 |
| PDMS-b-PEG | + | + | n.i. a | 40 ± 20 |
| PCL-b-PEG | − | + | n.i. a | 1.4 ± 0.9 |
| Block Copolymer | D0 × 107 [cm2/s] a | Rh [nm] a | dn/dc [cm3/g] b | Mw × 10−6 [g/mol] c | A2 × 105 [cm3mol/g2] c |
|---|---|---|---|---|---|
| PS-b-PEG | 1.8 ± 0.1 | 13.7 ± 0.4 | 0.150 | 1.1 ± 0.3 | 2.7 ± 2.8 |
| PMMA-b-PEG | 2.5 ± 0.4 | 10.5 ± 1.6 | 0.109 | 2.0 ± 0.9 | 5.2 ± 2.3 |
| PBd-b-PEG | 1.9 ± 0.2 | 12.9 ± 0.9 | 0.145 | 1.4 ± 0.5 | 6.9 ± 5.6 |
| PE-b-PEG | 1.4 ± 0.2 | 17.0 ± 0.8 | 0.114 | 3.0 ± 0.9 | 2.3 ± 0.8 |
| PDMS-b-PEG | 1.8 ± 0.2 | 13.7 ± 0.9 | 0.064 | 1.7 ± 0.9 | 1.0 ± 0.9 |
| PCL-b-PEG | 1.6 ± 0.1 | 15.4 ± 0.9 | 0.116 | 2.4 ± 0.9 | 1.8 ± 0.6 |
| Block Copolymer | [η] a [cm3/g] | [s] × 1015 [g/cm] a | D0 (DLS) × 107 [cm2/s] | MsD × 10−6 [g/mol] | Nagg b | A0 × 1010 |
|---|---|---|---|---|---|---|
| PS-b-PEG | 12 | 58.1 | 1.8 ± 0.1 | 0.88 | 75 | 2.2 |
| PMMA-b-PEG | 3 | 99.9 | 2.5 ± 0.4 | 1.11 | 80 | 3.2 |
| PBd-b-PEG | 10 | 78 | 1.9 ± 0.2 | 1.14 | 120 | 2.4 |
| PE-b-PEG | 10 | 101 | 1.4 ± 0.2 | 1.96 | 470 | 2.2 |
| PDMS-b-PEG | 4 | 56.4 | 1.8 ± 0.2 | 0.87 | 150 | 2.1 |
| PCL-b-PEG | 4 | 116 | 1.6 ± 0.1 | 2.01 | 220 | 2.5 |
| Block Copolymer | D [g/cm3] [41] | Rh [nm] a | Rcore [nm] b | φcore | Rcorona [nm] c |
|---|---|---|---|---|---|
| PS-b-PEG | 0.96–1.05 | 13.7 ± 0.4 | 4.7 ± 0.9 | 0.05 | 9.0 ± 1.3 |
| PMMA-b-PEG | 1.18 | 10.5 ± 1.6 | 5.3 ± 1.0 | 0.16 | 5.2 ± 2.6 |
| PBd-b-PEG | 0.889 | 12.9 ± 0.9 | 6.4 ± 1.2 | 0.12 | 6.5 ± 2.1 |
| PE-b-PEG | 0.88–0.97 | 17.0 ± 0.8 | 6.1 ± 1.2 | 0.04 | 10.9 ± 3.2 |
| PDMS-b-PEG | 0.965 | 13.7 ± 0.9 | 4.4 ± 0.9 | 0.03 | 9.3 ± 1.8 |
| PCL-b-PEG | 1.145 | 15.4 ± 0.9 | 7.4 ± 1.5 | 0.10 | 8.0 ± 2.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elistratova, A.A.; Gubarev, A.S.; Lezov, A.A.; Vlasov, P.S.; Solomatina, A.I.; Liao, Y.-C.; Chou, P.-T.; Tunik, S.P.; Chelushkin, P.S.; Tsvetkov, N.V. Amphiphilic Diblock Copolymers Bearing Poly(Ethylene Glycol) Block: Hydrodynamic Properties in Organic Solvents and Water Micellar Dispersions, Effect of Hydrophobic Block Chemistry on Dispersion Stability and Cytotoxicity. Polymers 2022, 14, 4361. https://doi.org/10.3390/polym14204361
Elistratova AA, Gubarev AS, Lezov AA, Vlasov PS, Solomatina AI, Liao Y-C, Chou P-T, Tunik SP, Chelushkin PS, Tsvetkov NV. Amphiphilic Diblock Copolymers Bearing Poly(Ethylene Glycol) Block: Hydrodynamic Properties in Organic Solvents and Water Micellar Dispersions, Effect of Hydrophobic Block Chemistry on Dispersion Stability and Cytotoxicity. Polymers. 2022; 14(20):4361. https://doi.org/10.3390/polym14204361
Chicago/Turabian StyleElistratova, Anastasiia A., Alexander S. Gubarev, Alexey A. Lezov, Petr S. Vlasov, Anastasia I. Solomatina, Yu-Chan Liao, Pi-Tai Chou, Sergey P. Tunik, Pavel S. Chelushkin, and Nikolai V. Tsvetkov. 2022. "Amphiphilic Diblock Copolymers Bearing Poly(Ethylene Glycol) Block: Hydrodynamic Properties in Organic Solvents and Water Micellar Dispersions, Effect of Hydrophobic Block Chemistry on Dispersion Stability and Cytotoxicity" Polymers 14, no. 20: 4361. https://doi.org/10.3390/polym14204361