Preparation and Laboratory Testing of Polymeric Scale Inhibitor Colloidal Materials for Oilfield Mineral Scale Control
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
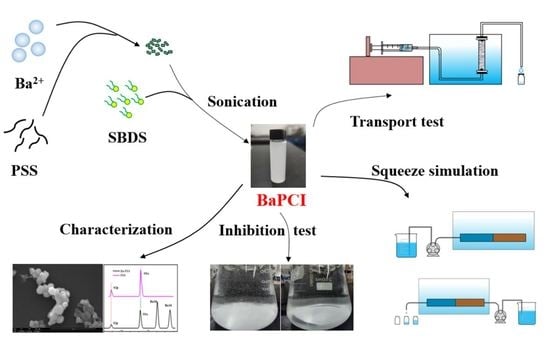
2.2. Preparation of BaPCI and Its Suspension
2.3. BaPCI Scale Inhibition Performance and Inhibition Efficacy Tests
2.4. BaPCI Column Transport Experiments
2.5. Laboratory Squeeze Simulation Experiments of BaPCI
2.6. Analytical and Characterization Methods
3. Results and Discussion
3.1. Optimization of Synthetic Conditions for BaPCI and Its Suspension
3.2. Characterization of the Prepared BaPCI and Its Suspension
3.3. Evaluation of Scale Inhibition Performance and Efficacy of the Prepared BaPCI Material
3.4. Transport of BaPCI in Calcite Packed Column
3.5. Laboratory Squeeze Simulation Experiments of BaPCI
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BaPCI | barium-PSS colloidal inhibitor |
| DI water | deionized water |
| DSC | differential scanning calorimetry |
| FT-IR | Fourier transform infrared |
| LSSE | laboratory squeeze simulation experiment |
| NSL | normalized squeeze lifetime |
| NTU | nephelometric turbidity unit |
| PSS | polystyrene sulfonate |
| PV | pore volume |
| SDBS | sodium dodecyl benzenesulfonate |
| SEM | scanning electron microscopy |
| SI | supporting information |
| SPD | scale protection duration |
| TE | transport experiment |
| TEM | transition electron microscopy |
| TGA | thermogravimetric analysis |
| XPS | X-ray photoelectron spectroscopy |
References
- Kan, A.T.; Dai, J.Z.; Deng, G.; Harouaka, K.; Lu, Y.-T.; Wang, X.; Zhao, Y.; Tomson, M. Recent advances in scale prediction: Approach and limitations. SPE J. 2019, 24, 2209–2220. [Google Scholar] [CrossRef]
- Kan, A.T.; Tomson, M.B. Scale prediction for oil and gas production. SPE J. 2012, 17, 362–378. [Google Scholar] [CrossRef]
- Fink, J. Petroleum Engineer’s Guide to Oil Field Chemicals and Fluids, 3rd ed.; Gulf Professional Publishing: Cambridge, MA, USA, 2021. [Google Scholar]
- Liu, Y.; Zhang, P. Review of phosphorus-based polymers for mineral scale and corrosion control in oilfield. Polymers 2022, 14, 2673. [Google Scholar] [CrossRef] [PubMed]
- Frenier, W.W.; Ziauddin, M. Formation, Removal, and Inhibition of Inorganic Scale in the Oilfield Environment; Society of Petroleum Engineers: Richardson, TX, USA, 2008. [Google Scholar]
- Hajirezaie, S.; Wu, X.; Soltanian, M.R.; Sakhad, S. Numerical simulation of mineral precipitation in hydrocarbon reservoirs and wellbores. Fuel 2019, 238, 462–472. [Google Scholar] [CrossRef]
- Chang, H.; Li, T.; Liu, B.; Vidic, R.D.; Elimelech, M.; Crittenden, J.C. Potential and implemented membrane-based technologies for the treatment and reuse of flowback and produced water from shale gas and oil plays: A review. Desalination 2019, 455, 34–57. [Google Scholar] [CrossRef]
- Kelland, M.A. Production Chemicals for the Oil and Gas Industry, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Zhang, P.; Kan, A.T.; Tomson, M.B. Oil field mineral scale control. In Mineral Scales and Deposits: Scientific and Technological Approaches; Amjad, Z., Demadis K, Eds.; Elsevier Publishing: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Sorbie, K.S.; Wesselingh, E.M.; Yuan, M.D.; Lemanczyk, R.Z.; Todd, A.C. Scale inhibitor squeeze strategies in horizontal wells. J. Can. Pet. Technol. 1997, 36, 27–35. [Google Scholar] [CrossRef]
- Tomson, M.B.; Kan, A.T.; Fu, G. Control of inhibitor squeeze via mechanistic understanding of inhibitor chemistry. SPE J. 2006, 11, 283–293. [Google Scholar] [CrossRef]
- Tomson, M.B.; Kan, A.T.; Fu, G.; Shen, D.; Nasr-El-Din, H.A.; Al-Saiari, H.; Al-Thubaiti, M.A. Mechanistic understanding of rock/phosphonate interactions and the effect of metal ions on inhibitor retention. SPE J. 2008, 13, 325–336. [Google Scholar] [CrossRef]
- Vazquez, O.; Mackay, E.; Sorbie, K. A two-phase near-wellbore simulator to model non-aqueous scale inhibitor squeeze treatments. J. Pet. Sci. Eng. 2012, 82, 90. [Google Scholar] [CrossRef]
- Kan, A.T.; Dai, Z.; Tomson, M.B. The state of the art in scale inhibitor squeeze treatment. Pet. Sci. 2020, 17, 1579–1601. [Google Scholar] [CrossRef]
- Sorbie, K.S.; Stamatiou, A. Analytical solutions of a one-dimensional linear model describing scale inhibitor precipitation treatments. Transp. Porous Media 2018, 123, 271–287. [Google Scholar] [CrossRef] [Green Version]
- Azari, V.; Vazquez, O.; Mackay, E.; Sorbie, K.; Jordan, M. Gradient descent algorithm to optimize the offshore scale squeeze treatments. J. Pet. Sci. Eng. 2022, 208, 109469. [Google Scholar] [CrossRef]
- Zhang, P. Review of synthesis and evaluation of inhibitor nanomaterials for oilfield mineral scale control. Front. Chem. 2020, 8, 576055. [Google Scholar] [CrossRef]
- Zhang, P.; Kan, A.T.; Tomson, M.B. Enhanced transport of novel crystalline phase calcium- phosphonate nanomaterials and their long term flow back performance in laboratory squeeze simulation tests. RSC Adv. 2016, 6, 5259–5269. [Google Scholar] [CrossRef]
- Franco-Aguirre, M.; Zabala, R.D.; Lopera, S.H.; Franco, C.A.; Cortés, F.B. Ca-DTPMP nanoparticles-based nanofluids for the inhibition and remediation of formation damage due to CaCO3 scaling in tight gas-condensate reservoirs. J. Pet. Sci. Eng. 2018, 169, 636–645. [Google Scholar] [CrossRef]
- Olayiwola, S.O.; Dejam, M. A comprehensive review on interaction of nanoparticles with low salinity water and surfactant for enhanced oil recovery in sandstone and carbonate reservoirs. Fuel 2019, 241, 1045–1057. [Google Scholar] [CrossRef]
- Ruan, G.; Kan, A.T.; Tomson, M.B.; Zhang, P. Facile one-pot synthesis of metal-phosphonate colloidal scale inhibitor: Synthesis and laboratory evaluation. Fuel 2020, 282, 118855. [Google Scholar] [CrossRef]
- Martínez-Sánchez, B.; Quintero-Jaime, A.F.; Huerta, F.; Cazorla-Amorós, D.; Morallón, E. Synthesis of phosphorus-containing polyanilines by electrochemical copolymerization. Polymers 2020, 12, 1029. [Google Scholar] [CrossRef]
- Li, S.; Guo, C.; Wang, X.; Guan, C.; Chen, G. Corrosion inhibition coating based on the self-assembled polydopamine films and its anti-corrosion properties. Polymers 2022, 14, 794. [Google Scholar] [CrossRef]
- Chen, J.; Chen, F.; Han, J.; Su, M.; Li, Y. Evaluation of scale and corrosion inhibition of modified polyaspartic acid. Chem. Eng. Technol. 2020, 43, 1048–1058. [Google Scholar] [CrossRef]
- Stamatakis, E.; Stubos, A.; Palyvos, J.; Chatzichristos, C.; Muller, J. An improved predictive correlation for the induction time of CaCO3 scale formation during flow in porous media. J. Colloid Interface Sci. 2005, 286, 7–13. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Kan, A.T.; Tomson, M.B. Mathematical inhibitor model for barium sulfate scale control. Langmuir 1996, 12, 1901–1905. [Google Scholar] [CrossRef]
- Xiao, J.; Kan, A.T.; Tomson, M.B. Prediction of BaSO4 precipitation in the presence and absence of a polymeric inhibitor: Phosphino-polycarboxylic acid. Langmuir 2001, 17, 4668–4673. [Google Scholar] [CrossRef]
- Mahrouqi, D.A.; Vinogradov, J.; Jackson, M.D. Zeta potential of artificial and natural calcite in aqueous solution. Adv. Colloid Interface Sci. 2017, 240, 60–76. [Google Scholar] [CrossRef] [Green Version]
- Kutluay, S. Excellent adsorptive performance of novel magnetic nano-adsorbent functionalized with 8-hydroxyquinoline-5-sulfonic acid for the removal of volatile organic compounds (BTX) vapors. Fuel 2021, 287, 119691. [Google Scholar] [CrossRef]
- Smith, B. Fundamentals of Fourier Transform Infrared Spectroscopy, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- De Morais, S.C.; Bezerra, B.G.P.; Castro, B.B.; Balaban, R.d.C. Evaluation of polyelectrolytic complexes based on poly(epichlorohydrin-co-dimethylamine) and poly (4-styrene-sulfonic acid-co-maleic acid) in the delivery of polyphosphates for the control of CaCO3 scale in oil wells. J. Mol. Liq. 2021, 339, 116757. [Google Scholar] [CrossRef]
- Yang, J.C.; Jablonsky, M.J.; Mays, J.W. NMR and FT-IR studies of sulfonated styrene-based homopolymers and copolymers. Polymer 2002, 43, 5125–5132. [Google Scholar] [CrossRef]
- Fang, M.; Kim, C.H.; Saupe, G.B.; Kim, H.; Waraksa, C.C.; Miwa, T.; Fujishima, A.; Mallouk, T.E. Layer-by-layer growth and condensation reactions of niobate and titanoniobate thin films. Chem. Mater. 1999, 11, 1526–1532. [Google Scholar] [CrossRef]
- Odobel, F.; Bujoli, B.; Massiot, D. Zirconium phosphonate frameworks covalently pillared with a bipyridine moiety. Chem. Mater. 2001, 13, 163–173. [Google Scholar] [CrossRef]
- Zhang, P.; Ruan, G.; Kan, A.T.; Tomson, M.B. Functional scale inhibitor nanoparticle capsule delivery vehicles for oilfield mineral scale control. RSC Adv. 2016, 6, 43016–43027. [Google Scholar] [CrossRef]
- Fan, C.; Kan, A.T.; Zhang, P.; Tomson, M.B. Barite nucleation and inhibition at 0 to 200 °C with and without thermodynamic hydrate inhibitors. SPE J. 2011, 16, 440–450. [Google Scholar] [CrossRef]
- Ryan, J.N.; Elimelech, M. Colloid mobilization and transport in groundwater. Colloids Surf. A 1996, 107, 1–56. [Google Scholar] [CrossRef]
- Kretzschmar, R.; Borkovec, M.; Grolimund, D.; Elimelech, M. Mobile subsurface colloids and their role in contaminant transport. Adv. Agron. 1999, 66, 121–193. [Google Scholar]
- Charbeneau, R.J. Groundwater Hydraulics and Pollutant Transport, 1st ed.; Waveland Press: Long Grove, IL, USA, 2006. [Google Scholar]
- Butler, E.L.; Reid, B.; Luckham, P.F.; Guldin, S.; Livingston, A.G.; Petit, C. Interparticle forces of a native and encapsulated metal-organic framework and their effects on colloidal dispersion. ACS Appl. Mater. Interfaces 2021, 13, 45898–45906. [Google Scholar] [CrossRef]
- Zhang, P.; Ruan, G.; Shen, D.; Kan, A.T.; Tomson, M.B. Transport and return of scale inhibitor reverse micelle nanofluid: Impact of preflush and overflush. RSC Adv. 2016, 6, 66672–66681. [Google Scholar] [CrossRef]
- Clark, M.M. Transport Modeling for Environmental Engineers and Scientists, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]








| Exp. # | Form of Inhibitor | Preflush Solution | Temp. °C | Flow Rate mL h−1 | Flow Vel. M d−1 | Calcite Size µm | |
|---|---|---|---|---|---|---|---|
| SDBS | KCl | ||||||
| TE #1 | PSS solution | 0 | 0.2% for all | 20 | 6.5 | 5.5 | 100–150 |
| TE #2 | BaPCI suspension for TE #2 to TE #10 | 0 | 20 | 6.5 | 5.5 | 100–150 | |
| TE #3 | 0.2 | 20 | 6.5 | 5.5 | 100–150 | ||
| TE #4 | 0.2 | 4 | 6.5 | 5.5 | 100–150 | ||
| TE #5 | 0.2 | 50 | 6.5 | 5.5 | 100–150 | ||
| TE #6 | 0.2 | 70 | 6.8 | 5.8 | 100–150 | ||
| TE #7 | 0.2 | 20 | 12.9 | 10.9 | 100–150 | ||
| TE #8 | 0.2 | 20 | 28.4 | 24 | 100–150 | ||
| TE #9 | 0.2 | 20 | 113 | 96 | 100–150 | ||
| TE #10 | 0.2 | 20 | 6.8 | 5.8 | 50–100 | ||
| Exp. # | Calculated Final C/C0 (%) * | Retardation Factor R | Deposition Coefficient Jd (min−1) | Dispersion Coefficient D (cm2 min−1) |
|---|---|---|---|---|
| TE #1 | 64.8 | 1.11 | 0.023 | 0.017 |
| TE #2 | 57.8 | 1.56 | 0.028 | 0.017 |
| TE #3 | 74.6 | 1.20 | 0.015 | 0.017 |
| TE #4 | 75.5 | 1.26 | 0.014 | 0.017 |
| TE #5 | 70.5 | 1.21 | 0.018 | 0.017 |
| TE #6 | 64.4 | 1.26 | 0.022 | 0.018 |
| TE #7 | 72.1 | 1.30 | 0.033 | 0.035 |
| TE #8 | 72.4 | 1.31 | 0.072 | 0.076 |
| TE #9 | 69.4 | 1.50 | 0.32 | 0.31 |
| TE #10 | 68.8 | 1.52 | 0.019 | 0.018 |
| Exp. # | Form of Inhibitor | Formation Materials | PV (mL) | Inh. Conc. (mg L−1) a | PSS Injected (mg) b | Squeeze Temp. (°C) | Flow Vel. (m d−1) |
|---|---|---|---|---|---|---|---|
| LSSE #1 | BaPCI | Calcite of 100–150 μm | 2.18 | 11,000 | 12.05 | 70 | 25.5 |
| LSSE #2 | PSS solution | 2.18 | 9,760 | 10.63 |
| Exp. # | Form of Inhibitor | PSS Returned (mg) a | Total % Returned b | Total Vol. Returned (PV) c | NSL (m3 kg−1) | SPD (d) |
|---|---|---|---|---|---|---|
| LSSE #1 | BaPCI | 10.05 | 83.4% | 840 | 152 | 101 |
| LSSE #2 | PSS solution | 10.39 | 97.8% | 20 | 4 | 2.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Dong, H.; Liu, X.; Zhang, P. Preparation and Laboratory Testing of Polymeric Scale Inhibitor Colloidal Materials for Oilfield Mineral Scale Control. Polymers 2022, 14, 4240. https://doi.org/10.3390/polym14194240
Wang H, Dong H, Liu X, Zhang P. Preparation and Laboratory Testing of Polymeric Scale Inhibitor Colloidal Materials for Oilfield Mineral Scale Control. Polymers. 2022; 14(19):4240. https://doi.org/10.3390/polym14194240
Chicago/Turabian StyleWang, Hanji, Huaxia Dong, Xianbin Liu, and Ping Zhang. 2022. "Preparation and Laboratory Testing of Polymeric Scale Inhibitor Colloidal Materials for Oilfield Mineral Scale Control" Polymers 14, no. 19: 4240. https://doi.org/10.3390/polym14194240