In-Situ Oxidative Polymerization of Pyrrole Composited with Cellulose Nanocrystal by Reactive Ink-Jet Printing on Fiber Substrates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Material
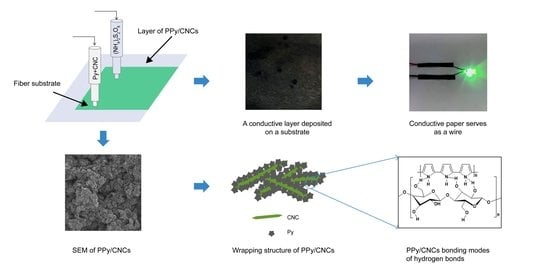
2.2. Deposition of PPy/CNCs Conductive Layer on the Substrate
2.3. Characteristics
3. Results and Discussion
3.1. Structure and Wrapping Structure of PPy/CNCs
3.2. Polarizing Optical Microscope Images of PPy/CNCs Deposited the Substrate
3.3. Surface Morphology
3.4. Organic Element Analysis
3.5. FTIR Spectra
3.6. Raman Spectra
3.7. Surface Resistance
3.7.1. The Surface Resistance of PPy/CNCs Deposited Different Fiber Substrates
3.7.2. Surface Resistance of PPy/CNCs Deposited PET Substrate
3.7.3. Effect of Different Sulfonates on Surface Resistance of PPy/CNCs Deposited on Paper Substrate
3.7.4. Paper-Based Device
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shirakawa, H.; Louis, E.J.; MacDiarmid, A.G.; Chiang, C.K.; Heeger, A.J. Synthesis of electrically conducting organic polymers: Halogen derivatives of polyacetylene, (CH)x. J. Chem. Soc. Chem. Commun. 1977, 16, 578–580. [Google Scholar] [CrossRef]
- Yu, L.; Yu, L.; Dong, Y.; Zhu, Y.; Fu, Y.; Ni, Q. Compressible polypyrrole aerogel as a lightweight and wideband electromagnetic microwave absorber. J. Mater. Sci. Mater. Electron. 2019, 30, 5598–5608. [Google Scholar] [CrossRef]
- Qu, C.; Zhao, P.; Wu, C.; Zhuang, Y.; Liu, J.; Li, W.; Liu, Z.; Liu, J. Electrospun PAN/PANI fiber film with abundant active sites for ultrasensitive trimethylamine detection. Sens. Actuators B Chem. 2021, 338, 129822. [Google Scholar] [CrossRef]
- Jia, X.; Chen, Z.; Duan, C.; Wang, Z.; Yin, Q.; Huang, F.; Cao, Y. Polythiophene derivatives compatible with both fullerene and non-fullerene acceptors for polymer solar cells. J. Mater. Chem. C 2018, 7, 314–323. [Google Scholar] [CrossRef]
- Zhao, H.; Hou, L.; Lu, Y. Electromagnetic shielding effectiveness and serviceability of the multilayer structured cuprammonium fabric/polypyrrole/copper (CF/PPy/Cu) composite. Chem. Eng. J. 2016, 297, 170–179. [Google Scholar] [CrossRef]
- Sajid, H.; Mahmood, T.; Ayub, K. High sensitivity of polypyrrole sensor for uric acid over urea, acetamide and sulfonamide: A density functional theory study. Synth. Met. 2018, 235, 49–60. [Google Scholar] [CrossRef]
- Chen, Y.; Cai, K.; Liu, C.; Song, H.; Yang, X. High-Performance and Breathable Polypyrrole Coated Air-Laid Paper for Flexible All-Solid-State Supercapacitors. Adv. Energy Mater. 2017, 7, 1701247. [Google Scholar] [CrossRef]
- Yussuf, A.; Al-Saleh, M.; Al-Enezi, S.; Abraham, G. Synthesis and Characterization of Conductive Polypyrrole: The Influence of the Oxidants and Monomer on the Electrical, Thermal, and Morphological Properties. Int. J. Polym. Sci. 2018, 2018, 4191747. [Google Scholar] [CrossRef] [Green Version]
- Li, T.; Zhou, Y.; Dou, Z.; Ding, L.; Dong, S.; Liu, N.; Qin, Z. Composite nanofibers by coating polypyrrole on the surface of polyaniline nanofibers formed in presence of phenylenediamine as electrode materials in neutral electrolyte. Electrochim. Acta 2017, 243, 228–238. [Google Scholar] [CrossRef]
- Lin, Q.; Yang, Y.; Ma, Y.; Zhang, R.; Wang, J.; Chen, X.; Shao, Z. Bandgap Engineered Polypyrrole–Polydopamine Hybrid with Intrinsic Raman and Photoacoustic Imaging Contrasts. Nano Lett. 2018, 18, 7485–7493. [Google Scholar] [CrossRef]
- Wang, X.-H.; Chen, X.-Q.; Peng, H.-S.; Wei, X.-F.; Cheng, K.; Liu, Y.-A.; Yang, W. Facile synthesis of polypyrrole–rhodamine B nanoparticles for self-monitored photothermal therapy of cancer cells. J. Mater. Chem. B 2019, 8, 1033–1039. [Google Scholar] [CrossRef] [PubMed]
- Hanif, Z.; Shin, D.; Choi, D.; Park, S.J. Development of a vapor phase polymerization method using a wet-on-wet process to coat polypyrrole on never-dried nanocellulose crystals for fabrication of compression strain sensor. Chem. Eng. J. 2019, 381, 122700. [Google Scholar] [CrossRef]
- Ropio, I.; Baptista, A.; Nobre, J.; Correia, J.; Belo, F.; Taborda, S.; Faustino, B.M.; Borges, J.; Kovalenko, A.; Ferreira, I. Cellulose paper functionalised with polypyrrole and poly(3,4-ethylenedioxythiophene) for paper battery electrodes. Org. Electron. 2018, 62, 530–535. [Google Scholar] [CrossRef]
- Lay, M.; González, I.; Tarrés, J.A.; Pellicer, N.; Bun, K.N.; Vilaseca, F. High electrical and electrochemical properties in bacterial cellulose/polypyrrole membranes. Eur. Polym. J. 2017, 91, 1–9. [Google Scholar] [CrossRef]
- Wang, N.; Li, G.; Yu, Z.; Zhang, X.; Qi, X. Conductive polypyrrole/viscose fiber composites. Carbohydr. Polym. 2015, 127, 332–339. [Google Scholar] [CrossRef]
- Wang, H.; Leaukosol, N.; He, Z.; Fei, G.; Si, C.; Ni, Y. Microstructure, distribution and properties of conductive polypyrrole/cellulose fiber composites. Cellulose 2013, 20, 1587–1601. [Google Scholar] [CrossRef]
- Dai, J.; Ogbeide, O.; Macadam, N.; Sun, Q.; Yu, W.; Li, Y.; Su, B.; Hasan, T.; Huang, X.; Huang, W. Printed gas sensors. Chem. Soc. Rev. 2020, 49, 1756–1789. [Google Scholar] [CrossRef]
- Zea, M.; Texidó, R.; Villa, R.; Borrós, S.; Gabriel, G. Specially Designed Polyaniline/Polypyrrole Ink for a Fully Printed Highly Sensitive pH Microsensor. ACS Appl. Mater. Interfaces 2021, 13, 33524–33535. [Google Scholar] [CrossRef]
- Li, Y.; Bober, P.; Apaydin, D.H.; Syrový, T.; Sariciftci, N.S.; Hromádková, J.; Sapurina, I.; Trchová, M.; Stejskal, J. Colloids of polypyrrole nanotubes/nanorods: A promising conducting ink. Synth. Met. 2016, 221, 67–74. [Google Scholar] [CrossRef]
- Hohnholz, D.; MacDiarmid, A.G. Line patterning of conducting polymers: New horizons for inexpensive, disposable electronic devices. Synth. Met. 2001, 121, 1327–1328. [Google Scholar] [CrossRef]
- Weng, B.; Shepherd, R.L.; Crowley, K.; Killard, A.J.; Wallace, G.G. Printing conducting polymers. Analyst 2010, 135, 2779–2789. [Google Scholar] [CrossRef] [PubMed]
- Stempien, Z.; Rybicki, T.; Kozanecki, M.; Szynkowska, M. In-situ deposition of polyaniline and polypyrrole electroconductive layers on textile surfaces by the reactive ink-jet printing technique. Synth. Met. 2015, 202, 49–62. [Google Scholar] [CrossRef]
- Blinova, N.V.; Stejskal, J.; Trchová, M.; Prokeš, J.; Omastová, M. Polyaniline and polypyrrole: A comparative study of the preparation. Eur. Polym. J. 2007, 43, 2331–2341. [Google Scholar] [CrossRef]
- Mao, H.; Dong, Y.; Qian, X.; An, X. Enhancement of bonding strength of polypyrrole/cellulose fiber (PPy/CF) hybrid through lignosulfonate doping. Cellulose 2017, 24, 2255–2263. [Google Scholar] [CrossRef]
- Cheng, Z.; Ma, Y.; Yang, L.; Cheng, F.; Huang, Z.; Natan, A.; Li, H.; Chen, Y.; Cao, D.; Huang, Z.; et al. Plasmonic-Enhanced Cholesteric Films: Coassembling Anisotropic Gold Nanorods with Cellulose Nanocrystals. Adv. Opt. Mater. 2019, 7, 1801816. [Google Scholar] [CrossRef]
- Al-Dulaimi, A.A.; Wanrosli, W.; Abdulrazak, L.F.; Husham, M. Preparation of nanocomposite polypyrrole/cellulose nanocrystals for conductive paper. Nord. Pulp Pap. Res. J. 2018, 33, 309–316. [Google Scholar] [CrossRef]
- Wei, C.; Fan, L.; Rao, W.; Bai, Z.; Xu, W.; Bao, H.; Xu, J. Electrothermochromic paper fabricated by depositing polypyrrole on one side. Cellulose 2017, 24, 5187–5196. [Google Scholar] [CrossRef]
- Bober, P.; Stejskal, J.; Šeděnková, I.; Trchová, M.; Martinková, L.; Marek, J. The deposition of globular polypyrrole and polypyrrole nanotubes on cotton textile. Appl. Surf. Sci. 2015, 356, 737–741. [Google Scholar] [CrossRef]
- Navik, R.; Shafiq, F.; Khan, A.; Datta, M.; Peng, X.; Kamruzzaman; Cai, Y. Preparation and characterizations of polypyrrole on liquid ammonia pre-treated wool fabric. Fibers Polym. 2017, 18, 1115–1123. [Google Scholar] [CrossRef]
- Hao, D.; Xu, B.; Cai, Z. Polypyrrole coated knitted fabric for robust wearable sensor and heater. J. Mater. Sci. Mater. Electron. 2018, 29, 9218–9226. [Google Scholar] [CrossRef]
- Bai, Y.; Liu, R.; Li, E.; Li, X.; Liu, Y.; Yuan, G. Graphene/Carbon Nanotube/Bacterial Cellulose assisted supporting for polypyrrole towards flexible supercapacitor applications. J. Alloys Compd. 2018, 777, 524–530. [Google Scholar] [CrossRef]
- Babayan, V.A.; Kazantseva, N.; Moučka, R.; Stejskal, J. Electromagnetic shielding of polypyrrole–sawdust composites: Polypyrrole globules and nanotubes. Cellulose 2017, 24, 3445–3451. [Google Scholar] [CrossRef]
- Devi, R.; Tapadia, K.; Maharana, T. Casting of carbon cloth enrobed polypyrrole electrode for high electrochemical performances. Heliyon 2020, 6, e03122. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, H.; Wang, Z.; Zhu, M.; Pei, Z.; Xue, Q.; Huang, Y.; Zhi, C. Nanostructured Polypyrrole as a flexible electrode material of supercapacitor. Nano Energy 2016, 22, 422–438. [Google Scholar] [CrossRef]
- Lizundia, E.; Nguyen, T.-D.; Vilas, J.L.; Hamad, W.Y.; MacLachlan, M.J. Chiroptical, morphological and conducting properties of chiral nematic mesoporous cellulose/polypyrrole composite films. J. Mater. Chem. A 2017, 5, 19184–19194. [Google Scholar] [CrossRef]
- Sun, Q.; Zhao, X.; Wang, D.; Dong, J.; She, D.; Peng, P. Preparation and characterization of nanocrystalline cellulose/Eucommia ulmoides gum nanocomposite film. Carbohydr. Polym. 2018, 181, 825–832. [Google Scholar] [CrossRef]
- Sharifi-Viand, A.; Mahjani, M.G.; Jafarian, M. Determination of fractal rough surface of polypyrrole film: AFM and electrochemical analysis. Synth. Met. 2014, 191, 104–112. [Google Scholar] [CrossRef]
- Wu, T.-M.; Chang, H.-L.; Lin, Y.-W. Synthesis and characterization of conductive polypyrrole with improved conductivity and processability. Polym. Int. 2009, 58, 1065–1070. [Google Scholar] [CrossRef]
- Kruer-Zerhusen, N.; Cantero-Tubilla, B.; Wilson, D.B. Characterization of cellulose crystallinity after enzymatic treatment using Fourier transform infrared spectroscopy (FTIR). Cellulose 2017, 25, 37–48. [Google Scholar] [CrossRef]
- Naduparambath, S.; Jinitha, T.V.; Shaniba, V.; Sreejith, M.P.; Balan, A.K.; Purushothaman, E. Isolation and characterisation of cellulose nanocrystals from sago seed shells. Carbohydr. Polym. 2018, 180, 13–20. [Google Scholar] [CrossRef]
- Ramesan, M.T.; Santhi, V. In situ synthesis, characterization, conductivity studies of polypyrrole/silver doped zinc oxide nanocomposites and their application for ammonia gas sensing. J. Mater. Sci. Mater. Electron. 2017, 28, 18804–18814. [Google Scholar] [CrossRef]
- Da Silva, F.A.G.; Alcaraz-Espinoza, J.J.; da Costa, M.M.; de Oliveira, H.P. Synthesis and characterization of highly conductive polypyrrole-coated electrospun fibers as antibacterial agents. Compos. Part B-Eng. 2017, 129, 143–151. [Google Scholar] [CrossRef]
- Parit, M.; Du, H.; Zhang, X.; Prather, C.; Adams, M.; Jiang, Z. Polypyrrole and cellulose nanofiber based composite films with improved physical and electrical properties for electromagnetic shielding applications. Carbohydr. Polym. 2020, 240, 116304. [Google Scholar] [CrossRef] [PubMed]
- Voronova, M.; Rubleva, N.; Kochkina, N.; Afineevskii, A.; Zakharov, A.; Surov, O. Preparation and Characterization of Polyvinylpyrrolidone/Cellulose Nanocrystals Composites. Nanomaterials 2018, 8, 1011. [Google Scholar] [CrossRef] [Green Version]
- Alves, A.P.P.; de Oliveira, L.P.; Castro, A.A.; Neumann, R.; de Oliveira, L.F.; Edwards, H.G.; Sant’Ana, A.C. The structure of different cellulosic fibres characterized by Raman spectroscopy. Vib. Spectrosc. 2016, 86, 324–330. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, S.; Ramaswamy, S.; Kim, Y.S.; Xu, F. Obtaining pure spectra of hemicellulose and cellulose from poplar cell wall Raman imaging data. Cellulose 2017, 24, 4671–4682. [Google Scholar] [CrossRef]
- Meng, Y.; Zhang, L.; Xing, R.; Huang, H.; Qu, Y.; Jiao, T.; Zhou, J.; Peng, Q. Facile preparation and electrochemical characterization of self-assembled core-shell diamond-polypyrrole nanocomposites. Colloids Surf. A Physicochem. Eng. Asp. 2018, 555, 787–794. [Google Scholar] [CrossRef]
- Chen, K.; Cao, M.; Qiao, Z.; He, L.; Wei, Y.; Ji, H.-F. Polymerization of solid state 2,2-bithiophene thin film or doped in cellulose paper using DBD plasma and its applications in paper-based electronics. ACS Appl. Polym. Mater. 2020, 2, 1518–1527. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, L.; Zhang, L.; Ge, S.; Yan, M.; Yu, J. In-situ synthesized polypyrrole-cellulose conductive networks for potential-tunable foldable power paper. Nano Energy 2017, 31, 174–182. [Google Scholar] [CrossRef]
- Das, M.; Sarkar, D. Development of room temperature ethanol sensor from polypyrrole (PPy) embedded in polyvinyl alcohol (PVA) matrix. Polym. Bull. 2017, 75, 3109–3125. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, W.; Jiang, Z.; Yu, D. Low temperature sintering nano-silver conductive ink printed on cotton fabric as printed electronics. Prog. Org. Coat. 2016, 101, 604–611. [Google Scholar] [CrossRef]
- Saravanan, C.; Shen, J.; Xiong, B.; Ni, Y. Role of stilbene-triazine sulfonic acid sodium salts in tuning electro-conductivity of polypyrrole-paper composites. Synth. Met. 2017, 228, 79–83. [Google Scholar] [CrossRef]
- Huang, L.; Yao, X.; Yuan, L.; Yao, B.; Gao, X.; Wan, J.; Zhou, P.; Xu, M.; Wu, J.; Yu, H.; et al. 4-Butylbenzenesulfonate modified polypyrrole paper for supercapacitor with exceptional cycling stability. Energy Storage Mater. 2017, 12, 191–196. [Google Scholar] [CrossRef]
- Mahmoodian, M.; Pourabbas, B.; Mohajerzadeh, S. Effect of anionic dopants on thickness, morphology and electrical properties of polypyrrole ultra-thin films prepared by in situ chemical polymerization. Thin Solid Films 2015, 583, 255–263. [Google Scholar] [CrossRef]
- Jafari, A.; Amini, A. Lactic acid gas sensor based on polypyrrole thin film. Mater. Lett. 2018, 236, 175–178. [Google Scholar] [CrossRef]















| Sample | C | N | S | C/N | C/S | N/S |
|---|---|---|---|---|---|---|
| PPy | 50.92 | 15.55 | 4.43 | 3.82 | 30.65 | 8.02 |
| PPy/CNCs | 53.85 | 14.67 | 4.191 | 4.28 | 34.30 | 8.01 |
| Sample | Py (g) | APS (g) | PVA (g) | Weight Ratio of Py/PVA | Conductivity (S cm−1) |
|---|---|---|---|---|---|
| Formula1 | 1.2 | 4.08 | 1.5 | 0.8:1 | 0.357 |
| Formula2 | 1.6 | 5.44 | 1 | 1.6:1 | 0.556 |
| Formula3 | 1.8 | 6.12 | 0.75 | 2.4:1 | 0.625 |
| Formula4 | 1.92 | 6.53 | 0.6 | 3.2:1 | 0.667 |
| Formula5 | 2 | 6.8 | 0.5 | 4:1 | 0.769 |
| Sulfonate | Chemical Formula | Optimum Doping Concentration | Conductivity (S cm−1) |
|---|---|---|---|
| p-TSA |  | 2% | 0.452 |
| SDBS |  | 1% | 0.492 |
| PSS |  | 1% | 0.813 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Cao, M.; Li, S.; Li, L.; Yang, Y.; Liu, R.; Sun, Z.; Mo, L.; Xin, Z.; Chen, Y.; et al. In-Situ Oxidative Polymerization of Pyrrole Composited with Cellulose Nanocrystal by Reactive Ink-Jet Printing on Fiber Substrates. Polymers 2022, 14, 4231. https://doi.org/10.3390/polym14194231
Li X, Cao M, Li S, Li L, Yang Y, Liu R, Sun Z, Mo L, Xin Z, Chen Y, et al. In-Situ Oxidative Polymerization of Pyrrole Composited with Cellulose Nanocrystal by Reactive Ink-Jet Printing on Fiber Substrates. Polymers. 2022; 14(19):4231. https://doi.org/10.3390/polym14194231
Chicago/Turabian StyleLi, Xu, Meijuan Cao, Shasha Li, Luhai Li, Yintang Yang, Ruping Liu, Zhicheng Sun, Lixin Mo, Zhiqing Xin, Yinjie Chen, and et al. 2022. "In-Situ Oxidative Polymerization of Pyrrole Composited with Cellulose Nanocrystal by Reactive Ink-Jet Printing on Fiber Substrates" Polymers 14, no. 19: 4231. https://doi.org/10.3390/polym14194231