Design of Acrylate-Terminated Polyurethane for Nylon Seamless Bonding Fabric Part I: Design of the End-Capping Thermoplastic Polyurethane Adhesive with Acrylate Copolymer
Abstract
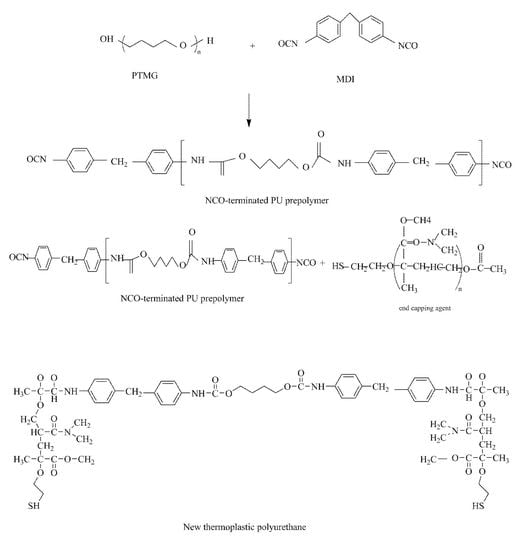
:1. Introduction
2. Experimental Method and Materials
2.1. Materials
2.2. Experimental Method
- (a)
- Bonding temperature: 150 °C.
- (b)
- Bonding pressure: 1.0 kg/cm2.
- (c)
- Bonding time: 20 s.
- (d)
- Standing time after bonding: 20 min for cooling and stabilization.
2.3. Instrumental Analysis
2.3.1. Test for Functional Group and H-Bond State of TPU
2.3.2. Test for Molecular Weight and Molecular Weight Distribution of TPU
2.3.3. Test for Pyrolysis Temperature of TPU
2.3.4. Test for Melting Point and Melting Enthalpy of TPU
2.3.5. Test for Glass Transition Temperature and Storage Modulus of TPU
3. Results and Discussion
3.1. Analysis of Functional Groups of TPU
3.2. Analysis of Molecular Weight and Distribution Coefficient of TPU
3.3. Analysis of Pyrolysis Temperature of TPU
3.4. Analysis of Melting Point and Melting Enthalpy of TPU
3.5. Analysis of Glass Transition Temperature and Storage Modulus of TPU
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Petrović, Z.S.; Milić, J.; Zhang, F.; Ilavsky, J. Fast-responding bio-based shape memory thermoplastic polyurethanes. Polymer 2017, 121, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Mi, H.Y.; Jing, X.; Hagerty, B.S.; Chen, G.; Huang, A.; Turng, L.S. Post-crosslinkable biodegradable thermoplastic polyurethanes: Synthesis, and thermal, mechanical, and degradation properties. Mater. Des. 2017, 127, 106–114. [Google Scholar] [CrossRef]
- Dang, L.N.; Le Hoang, S.; Malin, M.; Weisser, J.; Walter, T.; Schnabelrauch, M.; Seppälä, J. Synthesis and characterization of castor oil-segmented thermoplastic polyurethane with controlled mechanical properties. Eur. Polym. J. 2016, 81, 129–137. [Google Scholar] [CrossRef]
- Sultan, W.; Busnel, J.P. Kinetic study of polyurethanes formation by using differential scanning calorimetry. J. Therm. Anal. Calorim. 2006, 83, 355–359. [Google Scholar] [CrossRef]
- Cordeiro, N.; Belgacem, M.N.; Gandini, A.; Neto, C.P. Urethanes and polyurethanes from suberin: 1. kinetic study. Ind. Crop. Prod. 1997, 6, 163–167. [Google Scholar] [CrossRef]
- Burel, F.; Feldman, A.; Bunel, C. Hydrogenated hydroxy-functionalized polyisoprene (H-HTPI) and isocyanurate of isophorone diisocyanates (I-IPDI): Reaction kinetics study using FTIR spectroscopy. Polymer 2005, 46, 15–25. [Google Scholar] [CrossRef]
- Cho, Y.K.; Hwang, S.H. Diisocyanate type effects on flexibility and coating performance of UV-curable hard coatings based on tetrafunctional urethane acrylates. Polym. Bull. 2018, 75, 5795–5807. [Google Scholar] [CrossRef]
- Król, P.; Galina, H.; Kaczmarski, K. A three-parameter kinetic model of formation of linear polyurethane from 2,4-toluenediisocyanate and butane-1,4-diol. Macromol. Theory Simul. 1999, 8, 129–136. [Google Scholar] [CrossRef]
- Eceiza, A.; de la Caba, K.; Kortaberria, G.; Gabilondo, N.; Marieta, C.; Corcuera, M.A.; Mondragon, I. Influence of molecular weight and chemical structure of soft segment in reaction kinetics of polycarbonate diols with 4, 4′-diphenylmethane diisocyanate. Eur. Polym. J. 2005, 41, 3051–3059. [Google Scholar] [CrossRef]
- Lingxiao, M.; Xiuzhong, Z.; Wanbin, Z.; Haitao, Z.; Jianyong, W.; Jianbo, Q. Study on the preparation and performance comparison of side-chain hydroxyl-terminated polybutadiene derivatives with narrowly molecular weight distribution used for polyurethane. Polym. Test 2021, 104, 107389. [Google Scholar]
- Kulkarni, P.; Ojha, U.; Wei, X.; Gurung, N.; Seethamraju, K.; Faust, R. Thermal and mechanical properties of polyisobutylene-based thermoplastic polyurethanes. J. Appl. Polym. Sci. 2013, 130, 891–897. [Google Scholar] [CrossRef]
- Schneider, N.S.; Matton, R.W. Thermal transition behavior of polybutadiene containing polyurethanes. Polym. Eng. Sci. 1979, 19, 1122–1128. [Google Scholar] [CrossRef]
- Nakamae, K.; Nishino, T.; Asaoka, S.; Sudaryanto. Microphase separation and surface properties of segmented polyurethane-Effect of hard segment content. Int. J. Adhes. Adhes. 1996, 16, 233–239. [Google Scholar] [CrossRef]
- Chung, Y.C.; Lee, B.H.; Choi, J.W.; Chun, B.C. The lateral cross-linking of polyurethane using grafted epichlorohydrin and the impact on the tensile and thermal properties. Adv. Polym. Technol. 2018, 37, 323–331. [Google Scholar] [CrossRef]
- Abdiyev, K.Z.; Toktarbay, Z.; Zhenissova, A.Z.; Zhursumbaeva, M.B.; Kainazarova, R.N.; Nuraje, N. The new effective flocculants-copolymers of N,N-dimethyl-N,N-diallyl-ammonium chloride and N,N-dimethylacrylamide. Colloids Surf. A Physicochem. Eng. Asp. 2015, 480, 228–235. [Google Scholar] [CrossRef]
- Tamate, R.; Hashimoto, K.; Horii, T.; Hirasawa, M.; Li, X.; Shibayama, M.; Watanabe, M. Self-healing micellar ion gels based on multiple hydrogen bonding. Adv. Mater. 2018, 30, 1802792. [Google Scholar] [CrossRef]
- Kang, K.S.; Jee, C.; Bae, J.H.; Jung, H.J.; Kim, B.J.; Huh, P. Effect of soft/hard segments in poly(tetramethylene glycol)-polyurethane for water barrier film. Prog. Org. Coat. 2018, 123, 238–241. [Google Scholar] [CrossRef]
- Nozaki, S.; Hirai, T.; Higaki, Y.; Yoshinaga, K.; Kojio, K.; Takahara, A. Effect of chain architecture of polyol with secondary hydroxyl group on aggregation structure and mechanical properties of polyurethane elastomer. Polymer 2017, 116, 423–428. [Google Scholar] [CrossRef]
- Weng, N.C.; Wu, C.F.; Tsen, W.C.; Wu, C.L.; Suen, M.C. Synthesis and properties of shape memory polyurethanes generated from schiff-base chain extender containing benzoyl and pyridyl rings. Des. Monomers Polym. 2018, 21, 55–63. [Google Scholar] [CrossRef]
- Kim, H.N.; Lee, D.W.; Ryu, H.; Song, G.S.; Lee, D.S. Preparation and characterization of isosorbide-based self-healable polyurethane elastomers with thermally reversible bonds. Molecules 2019, 24, 1061. [Google Scholar] [CrossRef]
- Fenouillot, F.; Rousseau, A.; Colomines, G.; Saint-Loup, R.; Pascault, J.P. Polymers from renewable 1,4:3,6-dianhydr-OHexitols (isosorbide, isomannide and isoidide): A review. Prog. Polym. Sci. 2010, 35, 578–622. [Google Scholar] [CrossRef]
- Akın, A.; Işıklan, N. Microwave assisted synthesis and characterization of sodium alginate-graft-poly (N,N′-dimethylacrylamide). Int. J. Biol. Macromol. 2016, 82, 530–540. [Google Scholar] [CrossRef] [PubMed]
- Tripathy, J.; Mishra, D.K.; Yadav, M.; Behari, K. Synthesis, characterization and applications of graft copolymer (Chitosan-g-N,N-dimethylacrylamide). Carbohydr. Polym. 2010, 79, 40–46. [Google Scholar] [CrossRef]
- Fang, C.; Jing, Y.; Zong, Y.; Lin, Z. Effect of N,N-dimethylacrylamide (DMA) on the comprehensive properties of acrylic latex pressure sensitive adhesives. Int. J. Adhes Adhes 2016, 71, 105–111. [Google Scholar] [CrossRef]
- Tobing, S.; Klein, A.; Sperling, L.H.; Petrasko, B. Effect of network morphology on adhesive performance in emulsion blends of acrylic pressure sensitive adhesives. J. Appl. Polym. Sci. 2001, 81, 2109–2117. [Google Scholar] [CrossRef]
- Saiani, A.; Daunch, W.A.; Verbeke, H.; Leenslag, J.W.; Higgins, J.S. Origin of multiple melting endotherms in a high hard block content polyurethane. 1. Thermodynamic investigation. Macromolecules 2001, 34, 9059–9068. [Google Scholar] [CrossRef]
- Zhou, R.J.; Burkhart, T. Thermal and mechanical properties of poly (ether ester)-based thermoplastic elastomer composites filled with TiO2 nanoparticles. J. Mater. Sci. 2011, 46, 2281–2287. [Google Scholar] [CrossRef]
- Kuo, C.F.J.; Chen, J.B.; Lin, P.H.; Yen, H.T. Hot-melt pressure-sensitive adhesive for seamless bonding of nylon fabric part I: Effect of a functional monomer. Text. Res. J. 2019, 89, 926–935. [Google Scholar] [CrossRef]
- Zen, A.; Saphiannikova, M.; Neher, D.; Grenzer, J.; Grigorian, S.; Pietsch, U.; Asawapirom, U.; Janietz, S.; Scherf, U.; Lieberwirth, I.; et al. Effect of molecular weight on the structure and crystallinity of poly(3-hexylthiophene). Macromolecules 2006, 39, 2162–2171. [Google Scholar] [CrossRef]
- Wang, W.; Chen, H.; Dai, Q.; Zhao, D.; Zhou, Y.; Wang, L.; Zeng, D. Thermally healable PTMG-based polyurethane elastomer with robust mechanical properties and high healing efficiency. Smart Mater. Struct. 2018, 28, 015008. [Google Scholar] [CrossRef]
- Joseph, A.P.; Christopher, M.C.; Christine, H.; Karl, W.H.; Newell, R.W. Elucidating the Physicochemical Basis of the Glass Transition Temperature in Linear Polyurethane Elastomers with Machine Learning. J. Phys. Chem. B 2020, 124, 9722–9733. [Google Scholar]
- Jing, X.; Mi, H.Y.; Huang, H.X.; Turng, L.S. Shape memory thermoplastic polyurethane (TPU)/poly (ε-caprolactone) (PCL) blends as self-knotting sutures. J. Mech. Behav. Biomed. Mater. 2016, 64, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Leigh, D.A.; Robertson, C.C.; Slawin, A.M.Z.; Thomson, P.I.T. AAAA-DDDD quadruple hydrogen-bond arrays featuring NH··· N and CH··· N hydrogen bonds. J. Am. Chem. Soc. 2013, 135, 9939–9943. [Google Scholar] [CrossRef] [PubMed]
- Puszka, A. Thermal and mechanical behavior of new transparent thermoplastic polyurethane elastomers derived from cycloaliphatic diisocyanate. Polymers 2018, 10, 537. [Google Scholar] [CrossRef] [PubMed]
- Timti, A.; Tarzia, A.; Passaro, A.; Angiul, R. Thermographic analysis of polyurethane foams integrated with phase change material designed for dynamic thermal insulation insulation in refrigerated transport. Appl. Eng. 2014, 70, 201–210. [Google Scholar]
- Sankar, R.M.; Meera, K.S.; Mandal, A.B.; Jaisankar, S.N. Thermoplastic polyurethane/single-walled carbon nanotube composites with low electrical resistance surfaces. High Perform. Polym. 2012, 25, 135–146. [Google Scholar] [CrossRef]


















| Code | End Capping Agent Synthesis Monomer | Molecular Weight Analysis of End Capping Agent | ||||||
|---|---|---|---|---|---|---|---|---|
| MMA (wt%) | DMAA (wt%) | EAC (wt%) | AIBN (phm) | ME (phm) | Mw (g/mole) | Mn (g/mole) | PDI (Mw/Mn) | |
| D03 | 37 | 3 | 60 | 0.4 | 30 | 1070 | 943 | 1.13 |
| D05 | 35 | 5 | 60 | 0.4 | 30 | 1032 | 928 | 1.10 |
| D07 | 33 | 7 | 60 | 0.4 | 30 | 1016 | 906 | 1.13 |
| D10 | 30 | 10 | 60 | 0.4 | 30 | 1013 | 896 | 1.12 |
| Sample Code | End-Capping Agent Model | PTMG (wt%) | MDI (wt%) | End-Capping Agent (phm) |
|---|---|---|---|---|
| 5D03 | D03 | 75 | 25 | 5 |
| 5D05 | D05 | 75 | 25 | 5 |
| 5D07 | D07 | 75 | 25 | 5 |
| 5D10 | D10 | 75 | 25 | 5 |
| 10D03 | D03 | 75 | 25 | 10 |
| 10D05 | D05 | 75 | 25 | 10 |
| 10D07 | D07 | 75 | 25 | 10 |
| 10D10 | D10 | 75 | 25 | 10 |
| 15D03 | D03 | 75 | 25 | 15 |
| 15D05 | D05 | 75 | 25 | 15 |
| 15D07 | D07 | 75 | 25 | 15 |
| 15D10 | D10 | 75 | 25 | 15 |
| Sample | Carbonyl Region | Amine Region | ||||||
|---|---|---|---|---|---|---|---|---|
| –C=O (H-bond) | –C=O (free) | –NH (H-bond) | –NH (free) | |||||
| Wave Number (cm−1) | Peak Area (%) | Wave Number (cm−1) | Peak Area (%) | Wave Number (cm−1) | Peak Area (%) | Wave Number (cm−1) | Peak Area (%) | |
| 10D3 | 1711 | 41.7 | 1732 | 58.3 | 3300 | 70.0 | 3440 | 30 |
| 10D5 | 1711 | 43.6 | 1732 | 56.4 | 3300 | 76.0 | 3440 | 24 |
| 10D7 | 1711 | 50.5 | 1732 | 49.5 | 3300 | 79.0 | 3440 | 21 |
| 10D10 | 1711 | 57.6 | 1732 | 42.4 | 3300 | 81.0 | 3440 | 19 |
| End-Capping Agent (phm) | DMMA | Mw (g/mol) | Mn (g/mol) | DPI (Mw/Mn) |
|---|---|---|---|---|
| 5 | 3.0 wt% | 82,000 | 35,520 | 2.31 |
| 10.0 wt% | 111,580 | 56,100 | 1.98 | |
| 10 | 3.0 wt% | 63,460 | 28,270 | 2.24 |
| 10.0 wt% | 91,540 | 47,020 | 1.94 | |
| 15 | 3.0 wt% | 52,480 | 27,960 | 1.88 |
| 10.0 wt% | 68,080 | 37,940 | 1.80 | |
| Code | End-Capping Agent (phm) | Mw (g/mol) | Mn (g/mol) | DPI (Mw/Mn) |
| D03 | 5 | 82,000 | 35,520 | 2.31 |
| 15 | 52,480 | 27,960 | 1.88 | |
| D05 | 5 | 86,810 | 41,530 | 2.10 |
| 15 | 55,080 | 26,020 | 2.12 | |
| D07 | 5 | 96,330 | 47,980 | 2.00 |
| 15 | 64,730 | 28,300 | 2.28 | |
| D10 | 5 | 111,580 | 56,100 | 1.98 |
| 15 | 68,080 | 37,940 | 1.80 |
| Sample ID | 5D03 | 5D05 | 5D07 | 5D10 |
|---|---|---|---|---|
| Td5 (°C) | 312 | 328 | 322 | 332 |
| Td10 (°C) | 330 | 343 | 336 | 346 |
| decomposition rate (%/°C) | 0.80 | 0.83 | 0.83 | 0.86 |
| Sample ID | 10D03 | 10D05 | 10D07 | 10D10 |
| Td5 (°C) | 308 | 312 | 320 | 325 |
| Td10 (°C) | 325 | 329 | 337 | 341 |
| decomposition rate (%/°C) | 0.76 | 0.70 | 0.80 | 0.90 |
| Sample ID | 15D03 | 15D05 | 15D07 | 15D10 |
| Td5 (°C) | 309 | 317 | 320 | 323 |
| Td10 (°C) | 324 | 335 | 337 | 342 |
| decomposition rate (%/°C) | 0.71 | 0.83 | 0.83 | 0.90 |
| Code | 5D03 | 5D05 | 5D07 | 5D10 |
|---|---|---|---|---|
| Tmh (°C) | 131 | 142 | 140 | 144 |
| ΔH (J/g) | 0.8 | 1.3 | 2.4 | 4.5 |
| Code | 10D03 | 10D05 | 10D07 | 10D10 |
| Tmh (°C) | 121 | 131 | 136 | 140 |
| ΔH (J/g) | 0.8 | 1.2 | 2.1 | 2.6 |
| Code | 15D03 | 15D05 | 15D07 | 15D10 |
| Tmh (°C) | -- | 133 | 137 | 137 |
| ΔH (J/g) | -- | 0.9 | 1.8 | 2.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.-B.; Lin, S.-Y.; Ahmad, N.; Kuo, C.-F.J. Design of Acrylate-Terminated Polyurethane for Nylon Seamless Bonding Fabric Part I: Design of the End-Capping Thermoplastic Polyurethane Adhesive with Acrylate Copolymer. Polymers 2022, 14, 4079. https://doi.org/10.3390/polym14194079
Chen J-B, Lin S-Y, Ahmad N, Kuo C-FJ. Design of Acrylate-Terminated Polyurethane for Nylon Seamless Bonding Fabric Part I: Design of the End-Capping Thermoplastic Polyurethane Adhesive with Acrylate Copolymer. Polymers. 2022; 14(19):4079. https://doi.org/10.3390/polym14194079
Chicago/Turabian StyleChen, Jiong-Bo, Sheng-Yu Lin, Naveed Ahmad, and Chung-Feng Jeffrey Kuo. 2022. "Design of Acrylate-Terminated Polyurethane for Nylon Seamless Bonding Fabric Part I: Design of the End-Capping Thermoplastic Polyurethane Adhesive with Acrylate Copolymer" Polymers 14, no. 19: 4079. https://doi.org/10.3390/polym14194079