Super-Hydrophobic Magnetic Fly Ash Coated Polydimethylsiloxane (MFA@PDMS) Sponge as an Absorbent for Rapid and Efficient Oil/Water Separation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
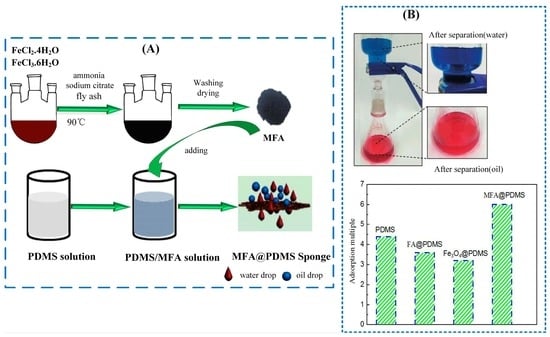
2.2. Preparation of Magnetic Fly Ash/Polydimethylsiloxane (MFA@PDMS) Sponge
2.3. Adsorption Capacity, Separation Efficiency and Reusability Test
2.4. Wenzel Model
2.5. Determination and Characterization
3. Results and Discussion
3.1. Materials Characterization
3.1.1. TEM Analysis of Nanometer Fe3O4
3.1.2. SEM Analysis
3.1.3. FT-IR Analysis
3.1.4. Characterization of Magnetic Properties on MFA@PDMS Sponge
3.2. Hydrophobicity of MFA@PDMS Sponge
3.3. Oil–Water Separation Performance of MFA@PDMS Sponge
3.3.1. Separation Performance for Immiscible Oil/Water
3.3.2. Separation Performance for Oil–Water Emulsion
3.4. Reuse of MFA@PDMS Sponge
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saleh, T.A.; Ali, I. Synthesis of polyamide grafted carbon microspheres for removal of rhodamine B dye and heavy metals. J. Environ. Chem. Eng. 2018, 6, 5361–5368. [Google Scholar] [CrossRef]
- Hoang, A.T.; Nguyen, X.P.; Duong, X.Q.; Huynh, T.T. Sorbent-based devices for the removal of spilled oil from water: A review. Environ. Sci. Pollut. Res. 2021, 28, 28876–28910. [Google Scholar] [CrossRef] [PubMed]
- Talimi, V.; Thodi, P.; Abdi, M.A.; Burton, R.; Liu, L.; Bruce, J. Enhancing harsh environment oil spill recovery using air floatation system. Saf. Extreme Environ. 2020, 2, 57–67. [Google Scholar] [CrossRef]
- Doshi, B.; Sillanpää, M.; Kalliola, S. A review of bio-based materials for oil spill treatment. Water Res. 2018, 135, 262–277. [Google Scholar] [CrossRef]
- Feng, L.; Li, S.H.; Li, Y.S.; Li, H.; Zhang, L.; Zhai, J.; Song, Y.; Liu, B.; Jiang, L.; Zhu, D. Super-hydrophobic surfaces: From natural to artificial. Adv. Mater. 2002, 14, 1857–1860. [Google Scholar] [CrossRef]
- Kesong, L.; Moyuan, C.; Akira, F.; Jiang, L. Bio-Inspired Titanium Dioxide Materials with Special Wettability and Their Applications. Chem. Rev. 2014, 114, 10044–10094. [Google Scholar]
- Shuto, W.; Kesong, L.; Xi, Y.; Jiang, L. Bioinspired surfaces with superwettability: New insight on theory, design, and applications. Chem. Rev. 2015, 115, 8230–8293. [Google Scholar]
- Hoang, A.T.; Nižetić, S.; Duong, X.Q.; Rowinski, L.; Nguyen, X.P. Advanced super-hydrophobic polymer-based porous absorbents for the treatment of oil-polluted water. Chemosphere 2021, 277, 130274. [Google Scholar] [CrossRef]
- Shami, Z.; Amininasab, S.M.; Katoorani, S.A.; Gharloghi, A.; Delbina, S. NaOH-Induced Fabrication of a Superhydrophilic and Underwater Superoleophobic Styrene-Acrylate Copolymer Filtration Membrane for Effective Separation of Emulsified Light Oil-Polluted Water Mixtures. Langmuir 2021, 37, 12304–12312. [Google Scholar] [CrossRef]
- Li, Z.; Wang, B.; Qin, X.; Wang, Y.; Liu, C.; Shao, Q.; Guo, Z. Superhydrophobic/superoleophilic polycarbonate/carbon nanotubes porous monolith for selective oil adsorption from water. ACS Sustain. Chem. Eng. 2018, 6, 13747–13755. [Google Scholar] [CrossRef]
- Li, X.M.; Reinhoudt, D.; Crego-Calama, M. What do we need for a superhydrophobic surface? A review on the recent progress in the preparation of superhydrophobic surfaces. Chem. Soc. Rev. 2007, 36, 1350–1368. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, R.N. Surface roughness and contact angle. J. Phys. Chem. 1949, 53, 1466–1467. [Google Scholar] [CrossRef]
- Baier, R.E. Surface behaviour of biomaterials: The theta surface for biocompatibility. J. Mater. Sci. Mater. Med. 2006, 17, 1057–1062. [Google Scholar] [CrossRef]
- Martin, S.; Bhushan, B. Transparent, wear-resistant, superhydrophobic and superoleophobic poly (dimethylsiloxane)(PDMS) surfaces. J. Colloid Interface Sci. 2017, 488, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Yu, C.; Cui, L.; Song, Z.; Zhao, X.; Ma, Y.; Jiang, L. Facile preparation of the porous PDMS oil-absorbent for oil/water separation. Adv. Mater. Interfaces 2017, 4, 1600862. [Google Scholar] [CrossRef]
- Chen, X.; Weibel, J.A.; Garimella, S.V. Continuous oil–water separation using polydimethylsiloxane-functionalized melamine sponge. Ind. Eng. Chem. Res. 2016, 55, 3596–3602. [Google Scholar] [CrossRef]
- Wang, J.C.; Li, Y.; Li, H.; Cui, Z.H.; Hou, Y.; Shi, W.; Jiang, K.; Qu, L.; Zhang, Y.P. A novel synthesis of oleophylic Fe2O3/polystyrene fibers by gamma-Ray irradiation for the enhanced photocatalysis of 4-chlorophenol and 4-nitrophenol degradation. J. Hazard. Mater. 2019, 379, 120806. [Google Scholar] [CrossRef]
- Wang, B.; Liu, Q.; Fan, Z.; Liang, T.; Tong, Q.; Fu, Y. Fabrication of PDMS/GA Composite Materials by Pickering Emulsion Method and Its Application for Oil-Water Separation. Energies 2021, 14, 5283. [Google Scholar] [CrossRef]
- Yang, R.L.; Zhu, Y.J.; Chen, F.F.; Qin, D.D.; Xiong, Z.C. Synthesis and structure of calcium silicate hydrate (CSH) modified by hydroxyl-terminated polydimethylsiloxane (PDMS). Constr. Build. Mater. 2021, 267, 120731. [Google Scholar]
- Yu, L.; Hao, G.; Gu, J.; Zhou, S.; Zhang, N.; Jiang, W. Fe3O4/PS magnetic nanoparticles: Synthesis, characterization and their application as sorbents of oil from waste water. J. Magn. Magn. Mater. 2015, 394, 14–21. [Google Scholar] [CrossRef]
- Javadian, S. Magnetic superhydrophobic polyurethane sponge loaded with Fe3O4@ oleic acid@ graphene oxide as high performance adsorbent oil from water. Chem. Eng. J. 2021, 408, 127369. [Google Scholar]
- Yang, R.L.; Zhu, Y.J.; Chen, F.F.; Qin, D.D.; Xiong, Z.C. Recyclable, fire-resistant, superhydrophobic, and magnetic paper based on ultralong hydroxyapatite nanowires for continuous oil/water separation and oil collection. ACS Sustain. Chem. Eng. 2018, 6, 10140–10150. [Google Scholar] [CrossRef]
- Zhang, Y.; Ren, F.; Liu, Y. A superhydrophobic EP/PDMS nanocomposite coating with high gamma radiation stability. Appl. Surf. Sci. 2018, 436, 405–410. [Google Scholar] [CrossRef]













Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, M.; Ma, X.; Chao, Y.; Chen, D.; Liao, Y. Super-Hydrophobic Magnetic Fly Ash Coated Polydimethylsiloxane (MFA@PDMS) Sponge as an Absorbent for Rapid and Efficient Oil/Water Separation. Polymers 2022, 14, 3726. https://doi.org/10.3390/polym14183726
Zhao M, Ma X, Chao Y, Chen D, Liao Y. Super-Hydrophobic Magnetic Fly Ash Coated Polydimethylsiloxane (MFA@PDMS) Sponge as an Absorbent for Rapid and Efficient Oil/Water Separation. Polymers. 2022; 14(18):3726. https://doi.org/10.3390/polym14183726
Chicago/Turabian StyleZhao, Mengqi, Xiaoqing Ma, Yuxi Chao, Dejun Chen, and Yinnian Liao. 2022. "Super-Hydrophobic Magnetic Fly Ash Coated Polydimethylsiloxane (MFA@PDMS) Sponge as an Absorbent for Rapid and Efficient Oil/Water Separation" Polymers 14, no. 18: 3726. https://doi.org/10.3390/polym14183726