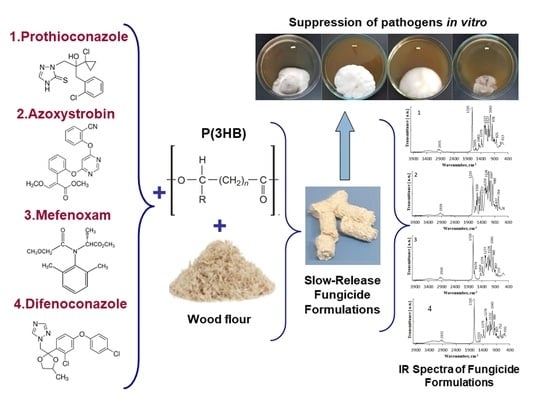
Degradable Poly(3-hydroxybutyrate)—The Basis of Slow-Release Fungicide Formulations for Suppressing Potato Pathogens
Abstract
:1. Introduction
2. Materials and Methods
2.1. Polymer
2.2. Fungicides
2.3. Constructing Embedded Fungicide Formulations
2.4. Physico-Chemical Properties of Embedded Fungicides
2.5. Degradation of Embedded Fungicides in Soil
2.6. Chromatographic Analysis of Fungicide Concentrations in Soil
2.7. A Microbiological Study
2.8. Testing Biological Activity of Embedded Fungicides
2.9. Statistics
3. Results and Discussion
3.1. Characterization of Fungicides
3.2. Characterization of Embedded Fungicides
3.3. Degradation Behavior of Fungicide Formulations in Soil and Release Kinetics of Active Ingredients
3.4. Biological Activity of Embedded Fungicides
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alan, K. Recent developments of safer formulations of agrochemicals. Environmentalist 2008, 28, 35–44. [Google Scholar]
- Carvalho, F.P.; Security, E. Pesticides, environment, and food safety. Food Energy Secur. 2017, 6, 48–60. [Google Scholar] [CrossRef]
- de Gomes, O.H.; Menezes, J.M.C.; da Costa, J.G.M.; Coutinho, H.D.M.; Teixeira, R.N.P.; do Nascimento, R.F. A socio-environmental perspective on pesticide use and food production. Ecotoxicol. Environ. Saf. 2020, 197, 110627. [Google Scholar] [CrossRef] [PubMed]
- Campos, E.V.R.; de Oliveira, J.L.; Fraceto, L.F. Applications of controlled release systems for fungicides, herbicides, acaricides, nutrients, and plant growth hormones: A review. Adv. Sci. Eng. Med. 2014, 6, 373–387. [Google Scholar] [CrossRef]
- Qaiss, A.; Bouhfi, R.; Essabir, H. Characterization and use of coir, almaund, apricot, argan, shell, and wood as reinforcement in the polymeric matrix in order to valorize these products. In Agricultural Biomass Based Potential Materials; Hakeem, R.H., Jawaid, M., Alothman., O.Y., Eds.; Springer: Cham, Switzerland, 2015; pp. 305–339. [Google Scholar]
- Singh, A.; Dhiman, N.; Kar, A.K.; Singh, D.; Purohit, M.P.; Ghosh, D.; Patnaik, S. Advances in controlled release pesticide formulations: Prospects to safer integrated pest management and sustainable agriculture. J. Hazard. Mater. 2020, 385, 121525. [Google Scholar] [CrossRef]
- Li, N.; Sun, C.; Jiang, J.; Wang, A.; Wang, C.; Shen, Y.; Huang, B.; An, C.; Cui, B.; Zhao, X.; et al. Advances in controlled-release pesticide formulations with improved efficacy and targetability. J. Agric. Food. Chem. 2021, 69, 12579–12597. [Google Scholar] [CrossRef]
- Chevillard, A.; Angellier-Coussy, H.; Guillard, V.; Gontard, N.; Gastaldi, E. Controlling pesticide release via structuring agropolymer and nanoclays based materials. J. Hazard. Mater. 2012, 205–206, 32–39. [Google Scholar] [CrossRef]
- Neri-Badang, M.C.; Chakraborty, S. Carbohydrate polymers as controlled release devices for pesticides. J. Carbohydr. Chem. 2019, 38, 67–85. [Google Scholar] [CrossRef]
- Luengo, J.M. Bioplastics from microorganisms. Cur. Opin. Microbiol. 2003, 6, 251–260. [Google Scholar] [CrossRef]
- Koller, M.; Maršálek, L.; de Sousa Dias, M.M.; Braunegg, G. Producing microbial polyhydroxyalkanoate (PHA) biopolyesters in a sustainable manner. New Biotechnol. 2017, 37, 24–38. [Google Scholar] [CrossRef]
- Shahid, S.; Razzaq, S.; Farooq, R.; Nazli, Z.H. Polyhydroxyalkanoates: Next generation natural biomolecules and a solution for the world’s future economy. Int. J. Biol. Macromol. 2021, 166, 297–321. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.Q. Plastics completely synthesized by bacteria: Polyhydroxyalkanoates. In Plastics from Bacteria. Natural Functions and Applications; Springer: Berlin/Heidelberg, Germany, 2010; pp. 17–37. [Google Scholar]
- Sudesh, K.; Abe, H. Practical Guide to Microbial Polyhydroxyalkanoates, 1st ed.; iSmithers Rapra Publishing: Shrewsbury, UK, 2010; 160p. [Google Scholar]
- Laycock, B.; Halley, P.; Pratt, S.; Werker, A.; Lant, P. The chemomechanical properties of microbial polyhydroxyalkanoates. Prog. Polym. Sci. 2013, 38, 536–583. [Google Scholar] [CrossRef]
- Volova, T.G.; Shishatskaya, E.I.; Sinskey, A.J. Degradable Polymers: Production, Properties, Applications; Nova Science Pub Inc.: New York, NY, USA, 2013; 380p. [Google Scholar]
- Chen, G.Q.; Chen, X.Y.; Wu, F.Q.; Chen, J.C. Polyhydroxyalkanoates (PHA) toward cost competitiveness and functionality. Adv. Ind. Eng. Polym. Res. 2020, 3, 1–7. [Google Scholar] [CrossRef]
- Tarrahi, R.; Fathi, Z.; Özgür, M.; Seydibeyoğlu, K.; Doustkhah, E.; Khataee, A. Polyhydroxyalkanoates (PHA): From production to nanoarchitecture. Int. J. Biol. Macromol. 2020, 146, 596–619. [Google Scholar] [CrossRef] [PubMed]
- Israni, N.; Shivakumar, S. Polyhydroxyalkanoates in packaging. In Biotechnological Applications of Polyhydroxyalkanoates; Kalia, V.C., Ed.; Springer Nature: Singapore, 2019; pp. 363–388. [Google Scholar]
- Koller, M.; Mukherjee, A. Polyhydroxyalkanoates–linking properties, applications, and end-of-life options. Chem. Biochem. Eng. Q. 2020, 34, 115–129. [Google Scholar] [CrossRef]
- Polyhydroxyalkanoate (PHA) Market by Type, Manufacturing Technology and Application. Global Forecast to 2021. Research and Markets. Available online: https://www.marketsandmarkets.com/Market-Reports/pha-market395.html (accessed on 26 June 2017).
- Adeleye, A.T.; Odoh, C.K.; Enudi, O.C.; Banjoko, O.O.; Osiboye, O.O.; Odediran, E.T.; Louis, H. Sustainable synthesis and applications of polyhydroxyalkanoates (PHAs) from biomass. Process Biochem. 2020, 96, 174–193. [Google Scholar] [CrossRef]
- Elmowafy, E.; Abdal-Hay, A.; Skouras, A.; Tiboni, M.; Casettari, L.; Guarino, V. Polyhydroxyalkanoate (PHA): Applications in drug delivery and tissue engineering. Expert Rev. Med. Devices 2019, 16, 467–482. [Google Scholar] [CrossRef]
- Jiang, G.; Hill, D.J.; Kowalczuk, M.; Johnston, B.; Adamus, G.; Irorere, V.; Radecka, I. Carbon sources for polyhydroxyalkanoates and an integrated biorefinery. Int. J. Mol. Sci. 2016, 17, 1157. [Google Scholar] [CrossRef]
- Maiti, P.; Batt, C.A.; Giannelis, E.P. Renewable plastics: Synthesis and properties of PHB nanocomposites. Polym. Mater. Sci. Eng. 2003, 88, 58–59. [Google Scholar]
- Torres-Giner, S.; Montanes, N.; Boronat, T.; Quiles-Carrillo, L.; Balart, R. Melt grafting of sepiolite nanoclay onto poly(3-hydroxybutyrate-co-4-hydroxybutyrate) by reactive extrusion with multi-functional epoxy-based styrene-acrylic oligomer. Eur. Polym. J. 2016, 84, 693–707. [Google Scholar] [CrossRef]
- Macedo, J.S.; Costa, M.F.; Travares, M.I.B.; Thirè, R.M.S.M. Preparation and characterization of composites based on polyhydroxybutyrate and waste powder from coconut fibers processing. Polym. Eng. Sci. 2010, 50, 1466–1475. [Google Scholar] [CrossRef]
- Gunning, M.A.; Geever, L.M.; Killion, J.A.; Lyons, J.G.; Higginbotha, C.L. Mechanical and biodegradation performance of short natural fibre polyhydroxybutyrate composites. Polym. Test. 2013, 32, 1603–1611. [Google Scholar] [CrossRef]
- Angelini, S.; Cerruti, P.; Immirzi, B.; Scarinzi, G.; Malinconico, M. Acid-insoluble lignin and holocellulose from a lignocellulosic biowaste: Bio-fillers in poly(3-hydroxybutyrate). Eur. Polym. J. 2016, 76, 63–76. [Google Scholar] [CrossRef]
- Iewkittayakorn, J.; Khunthongkaew, P.; Chotigeat, W.; Sudesh, K. Effect of microwave pretreatment on the properties of particleboard made from para rubber wood sawdust with the addition of polyhydroxyalkanoates. Sains Malays. 2017, 46, 1361–1367. [Google Scholar] [CrossRef]
- Vandi, L.J.; Chan, C.M.; Werker, A.; Richardson, D.; Laycock, B.; Pratt, S. Wood-PHA composites: Mapping opportunities. Polymers 2018, 10, 751. [Google Scholar] [CrossRef]
- Melendez-Rodriguez, B.; Torres-Giner, S.; Aldureid, A.; Cabedo, L.; Lagaron, J.M. Reactive melt mixing of poly(3-hydroxybutyrate)/rice husk flour composites with purified biosustainably produced poly(3-hydroxybutyrate-co-3-hydroxyvalerate). Materials 2019, 12, 2152. [Google Scholar] [CrossRef]
- Chen, G.Q. Polyhydroxyalkanoates. In Biodegradable Polymers for Industrial Applications; Smith, R., Ed.; CRC Press: Cambridge, UK, 2005; pp. 32–56. [Google Scholar]
- Satyanarayana, K.G.; Arizaga, G.G.C.; Wypych, F. Biodegradable composites based on lignocellulosic fibers—An overview. Prog. Polym. Sci. 2009, 34, 982–1021. [Google Scholar] [CrossRef]
- Thompson, D.N.; Emerick, R.W.; England, A.B.; Flanders, J.P.; Loge, F.J.; Wiedeman, K.A.; Wolcott, M.P. Final Report: Development of Renewable Microbial Polyesters for Cost Effective and Energy-Efficient Wood-Plastic Composites; Idaho National Laboratory: Idaho Falls, ID, USA, 2010; p. 83415.
- Gregorova, A.; Wimmer, R.; Hrabalova, M.; Koller, M.; Ters, T.; Mundigler, N. Effect of surface modification of beech wood flour on mechanical and thermal properties of poly (3-hydroxybutyrate)/wood flour composites. Holzforschung 2009, 63, 565–570. [Google Scholar] [CrossRef]
- Srubar, W.V.; Pilla, S.; Wright, Z.C.; Ryan, C.A.; Greene, J.P.; Frank, C.W.; Billington, S.L. Mechanisms and impact of fiber-matrix compatibilization techniques on the material characterization of PHBV/oak wood flour engineered biobased composites. Compos. Sci. Technol. 2012, 72, 708–715. [Google Scholar] [CrossRef]
- Chan, C.M.; Pratt, S.; Halley, P.; Richardson, D.; Werkera, A.; Laycock, B.; Vandi, L.-J. Mechanical and physical stability of polyhydroxyalkanoate (PHA)-based wood plastic composites (WPCs) under natural weathering. Polym. Test. 2019, 73, 214–221. [Google Scholar] [CrossRef]
- Chan, C.M.; Vandi, L.-J.; Pratt, S.; Halley, P.; Ma, Y.; Chen, G.-Q.; Richardson, D.; Werker, A.; Laycock, B. Understanding the effect of copolymer content on the processability and mechanical properties of polyhydroxyalkanoate (PHA)/wood composites. Compos. Part A Appl. Sci. Manuf. 2019, 214, 105437. [Google Scholar] [CrossRef]
- Chan, C.M.; Vandi, L.-J.; Pratt, S.; Halley, P.; Richardson, D.; Werker, A.; Laycock, B. Insights into the biodegradation of PHA/wood composites: Micro- and macroscopic changes. Sustain. Mater. Technol. 2019, 21, e00099h. [Google Scholar] [CrossRef] [Green Version]
- Bugnicourt, E.; Cinelli, P.; Lazzeri, A.; Alvarez, V. Polyhydroxyalkanoate (PHA): Review of synthesis, characteristics, processing and potential applications in packaging. Express Polym. Lett. 2014, 8, 791–808. [Google Scholar] [CrossRef]
- Muizniece-Brasava, S.; Dukalska, L. Impact of biodegradable PHB packaging composite materials on dairy product quality. LLU Raksti 2006, 16, 79–87. [Google Scholar]
- Keshavarz, T.; Roy, I. Polyhydroxyalkanoates: Bioplastics with a green agenda. Curr. Opin. Microbiol. 2010, 13, 321–326. [Google Scholar] [CrossRef]
- Kanekar, P.P.; Kulkarni, S.O.; Nilegaonkar, S.S.; Sarnaik, S.S.; Kshirsagar, P.R.; Jog, J.P. Microbial biodegradable polymer having potential application in packaging. In Proceedings of the First Indo-US International Conference on Polymers for Packaging Applications (ICPPA), Kottayam, India, 31 March–2 April 2012. [Google Scholar]
- Arrieta, M.P.; López, J.; Hernández, A.; Rayón, E. Ternary PLA-PHB-Limonene blends intended for biodegradable food packaging applications. Eur. Polym. J. 2014, 50, 255–270. [Google Scholar] [CrossRef]
- Popović, S.Z.; Lazić, V.L.; Hromiš, N.M.; Šuput, D.Z.; Bulut, S.N. Biopolymer packaging materials for food shelf-life prolongation. In Biopolymers for Food Design. Handbook of Food Bioengineering; Grumezescu, A.M., Holban, A.M., Eds.; Academic Press: London, UK, 2018; Volume 20, pp. 223–277. [Google Scholar]
- Kalia, V.C.; Patel, S.K.S.; Shanmugam, R.; Lee, J.K. Polyhydroxyalkanoates: Trends and advances toward biotechnological applications. Bioresour. Technol. 2021, 326, 124737. [Google Scholar] [CrossRef]
- Poltronieri, P.; Kumar, P. Polyhydroxyalkanoates (PHAs) in industrial applications. In Handbook of Ecomaterials; Martínez, L.M.T., Kharissova, O.V., Kharisov, B.I., Eds.; Springer: Cham, Switzerland, 2019; pp. 2843–2872. [Google Scholar]
- Tleuova, A.B.; Wielogorska, E.; Talluria, P.; Štěpánek, F.; Elliott, C.T.; Grigoriev, D.O. Recent advances and remaining barriers to producing novel formulations of fungicides for safe and sustainable agriculture. J. Control. Release 2020, 326, 468–481. [Google Scholar] [CrossRef]
- Dhananjayan, V.; Jayakumar, S.; Ravichandran, B. Conventional methods of pesticide application in agricultural field and fate of the pesticides in the environment and human health. In Controlled Release of Pesticides for Sustainable Agriculture; Rakhimol, K.R., Thomas, S., Volova, T., Jayachandran, K., Eds.; Springer: Cham, Switzerland, 2020; pp. 1–39. [Google Scholar]
- Savenkova, L.; Gercberga, Z.; Muter, O.; Nikolaeva, V.; Dzene, A.; Tupureina, V. PHB-based films as matrices for pesticides. Process Biochem. 2002, 37, 719–722. [Google Scholar] [CrossRef]
- Grillo, R.; de Melo, N.F.S.; de Lima, R.; Lourenço, R.W.; Rosa, A.H.; Fraceto, L.F. Characterization of atrazine-loaded biodegradable poly (hydroxybutyrate-co-hydroxyvalerate) microspheres. J. Polym. Environ. 2010, 18, 26–32. [Google Scholar] [CrossRef]
- Grillo, R.; Pereira, A.D.E.S.; de Melo, N.F.S.; Porto, R.M.; Feitosa, L.O.; Tonello, P.S.; Filho, N.L.D.; Rosa, A.H.; Lima, R.; Fraceto, L.F. Controlled release system for ametryn using polymer microspheres: Preparation, characterization and release kinetics in water. J. Hazard. Mater. 2011, 186, 1645–1651. [Google Scholar] [CrossRef] [PubMed]
- Lobo, F.A.; de Aguirre, C.L.; Silva, M.S.; Grillo, R.; de Melo, N.F.S.; de Oliveira, L.K.; de Morais, L.C.; Campos, V.; Rosa, A.H.; Fraceto, L.F. Poly (hydroxybutyrate-co-hydroxyvalerate) microspheres loaded with atrazine herbicide: Screening of conditions for preparation, physico-chemical characterization, and in vitro release studies. Polym. Bull. 2011, 67, 479–495. [Google Scholar] [CrossRef]
- Agustien, A.; Sari, A.; Fitria, A.; Djamaan, A. Manufacture of a slow release herbicide of methyl-metsulfuron using biopolymer of poly (3-hydroxybutyrate) as matrix. Der Pharma Chem. 2016, 8, 105–111. [Google Scholar]
- Kwiecien, I.; Adamus, G.; Jiang, G.; Radecka, I.; Baldwin, T.C.; Khan, H.R.; Johnston, B.; Pennetta, V.; Hill, D.; Bretz, I.; et al. Biodegradable PBAT/PLA blend with bioactive MCPA-PHBV conjugate suppresses weed growth. Biomacromolecules 2018, 19, 511–520. [Google Scholar] [CrossRef]
- Feng, Z.F.; Duan, T.T.; Liao, G.H.; Cao, L.D.; Cao, C.; Li, F.M.; Chen, C.; Huang, Q.L. Preparation of Fenoxanil Micro-Capsule. In Proceedings of the Academic Annual Conference Sponsored by the Chinese Society of Plant Protection in 2018, Xi’an, China, 25–27 November 2018; pp. 213–219. [Google Scholar]
- Khan, H.; Kaur, S.; Baldwin, T.C.; Radecka, I.; Jiang, G.; Bretz, I.; Duale, K.; Adamus, G.; Kowalczuk, M. Effective control against broadleaf weed species provided by biodegradable PBAT/PLA mulch film embedded with the herbicide 2-methyl-4-chlorophenoxyacetic acid (MCPA). ACS Sustain. Chem. Eng. 2020, 8, 5360–5370. [Google Scholar] [CrossRef]
- Chen, G.; Cao, L.; Cao, C.; Zhao, P.; Li, F.; Xu, B.; Huang, Q. Effective and sustained control of soil-borne plant diseases by biodegradable polyhydroxybutyrate mulch films embedded with fungicide of prothioconazole. Molecules 2021, 26, 762. [Google Scholar] [CrossRef]
- Volova, T.G.; Shishatskaya, E.I.; Prudnikova, S.V.; Zhila, N.O.; Boyandin, A.N. New Generation Formulations of Agrochemicals: Current Trends and Future Priorities; Apple Academic Press Inc.: Oakville, ON, Canada, 2020; 286p. [Google Scholar]
- Volova, T.G.; Baranoskiy, S.V.; Demidenko, A.V.; Zhila, N.O.; Kiselev, E.G.; Prudnikova, S.V.; Sukovatiy, A.G.; Shishatskaya, E.I.; Shumilova, A.A. Long-Acting Pesticidal Agent for Soil Application. RF Patent for Invention 2733295, 23 March 2020. [Google Scholar]
- Thomas, S.; Shumilova, A.A.; Kiselev, E.G.; Baranovsky, S.V.; Vasiliev, A.D.; Kuzmin, E.G.; Nemtsev, I.V.; Sukovatyi, A.G.; Avinash, R.P.; Volova, T.G. Thermal, mechanical and biodegradation studies of biofiller based poly-3-hydroxybutyrate biocomposites. Int. J. Biol. Macromol. 2020, 155, 1373–1384. [Google Scholar] [CrossRef]
- Kiselev, E.G.; Boyandin, A.N.; Zhila, N.O.; Prudnikova, S.V.; Shumilova, A.A.; Baranovskiy, S.V.; Shishatskaya, E.I.; Thomas, S.; Volova, T.G. Constructing sustained-release herbicide formulations based on poly-3-hydroxybutyrate and natural materials as a degradable matrix. Pest Manag. Sci. 2020, 76, 1772–1785. [Google Scholar] [CrossRef]
- Volova, T.G.; Prudnikova, S.V.; Zhila, N.O. Fungicidal activity of slow-release P(3HB)/TEB formulations in wheat plant communities infected by Fusarium moniliforme. Environ. Sci. Pollut. Res. 2018, 25, 552–561. [Google Scholar] [CrossRef]
- Volova, T.; Prudnikova, S.; Boyandin, A.; Zhila, N.; Kiselev, E.; Shumilova, A.; Baranovskiy, S.; Demidenko, A.; Shishatskaya, E.; Thomas, S. Constructing slow-release fungicide formulations based on poly (3-hydroxybutyrate) and natural materials as a degradable matrix. J. Agric. Food Chem. 2019, 67, 9220–9231. [Google Scholar] [CrossRef]
- Murueva, A.V.; Shershneva, A.M.; Abanina, K.V.; Prudnikova, S.V.; Shishatskaya, E.I. Development and characterization of ceftriaxone-loaded P3HB-based microparticles for drug delivery. Dry. Technol. 2019, 37, 1131–1142. [Google Scholar] [CrossRef]
- Volova, T.; Baranovsky, S.; Petrovskaya, O.; Shumilova, A.; Sukovatyi, A. Biological effects of the free and embedded metribuzin and tribenuron-methyl herbicides on various cultivated weed species. J. Environ. Sci. Health Part B 2020, 55, 1009–1019. [Google Scholar] [CrossRef] [PubMed]
- Volova, T.G.; Shumilova, A.A.; Zhila, N.O.; Sukovatyi, A.G.; Shishatskaya, E.I.; Thomas, S. Efficacy of slow-release formulations of metribuzin and tribenuron methyl herbicides for controlling weeds of various species in wheat and barley stands. ACS Omega 2020, 5, 25135–25147. [Google Scholar] [CrossRef] [PubMed]
- Volova, T.G.; Demidenko, A.V.; Kurachenko, N.L.; Baranovsky, S.V.; Petrovskaya, O.D.; Shumilova, A.A. Efficacy of embedded metribuzin and tribenuron-methyl herbicides in field-grown vegetable crops infested by weeds. Environ. Sci. Pollut. Res. 2021, 28, 982–994. [Google Scholar] [CrossRef] [PubMed]
- Volova, T.G.; Kurachenko, N.L.; Bopp, V.L.; Thomas, S.; Demidenko, A.V.; Kiselev, E.G.; Baranovsky, S.V.; Sukovatyi, A.G.; Zhila, N.O.; Shishatskaya, E.I. Assessment of the efficacy of slow-release formulations of the tribenuron-methyl herbicide in field-grown spring wheat. Environ. Sci. Pollut. Res. 2021, 29, 20249–20264. [Google Scholar] [CrossRef]
- Boyandin, A.N.; Kazantseva, E.A. Constructing slow-release formulations of herbicide metribuzin using its co-extrusion with biodegradable polyester poly-ε-caprolactone. J. Environ. Sci. Health Part B 2021, 56, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Vijayamma, R.; Maria, H.J.; Thomas, S.; Shishatskaya, E.I.; Kiselev, E.G.; Nemtsev, I.V.; Sukhanova, A.A.; Volova, T.G. A study of the properties and efficacy of microparticles based on P(3HB) and P(3HB/3HV) loaded with herbicides. J. Appl. Polym. Sci. 2021, 139, e51756. [Google Scholar] [CrossRef]
- Goutam, U.; Thakur, K.; Salaria, N.; Kukreja, S. Recent approaches for late blight disease management of potato caused by Phytophthora infestans. In Fungi and Their Role in Sustainable Development: Current Perspectives; Gehlot, P., Singh, J., Eds.; Springer: Singapore, 2018; pp. 311–325. [Google Scholar]
- Campos, H.; Ortiz, O. The Potato Crop: Its Agricultural, Nutritional and Social Contribution to Humankind; Springer: Cham, Switzerland, 2020; 307p. [Google Scholar]
- Ali, M.; Parmar, A.; Niedbała, G.; Wojciechowski, T.; El-Yazied, A.A.; El-Gawad, H.; Nahhas, N.; Ibrahim, M.; El-Mogy, M. Improved shelf-life and consumer acceptance of fresh-cut and fried potato strips by an edible coating of garden cress seed mucilage. Foods 2021, 10, 1536. [Google Scholar] [CrossRef]
- Karpukhin, M.Y.; Keita, F. Potato diseases and measures to control them in the conditions of the middle Urals. E3S Web Conf. 2020, 222, 03022. [Google Scholar] [CrossRef]
- Volova, T.G.; Shishatskaya, E.I. Cupriavidus eutrophus Bacterial Strain VKPM B-10646-A Producer of Polyhydroxyalkanoates and a Method of Their Production (Cupriavidus eutrophus Shtamm Bakterii VKPM B-10646-Produtsent Poligidroksialkanoatov i Sposob Ikh Polucheniya. RF Patent 2439143, 10 January 2012. (In Russian). [Google Scholar]
- Nikolaeva, N.I.; Tereshkova, L.P.; Chkhvirkiya, E.G.; Bereznyak, I.V.; Sinitskaya, T.A.; Busova, T.N.; Gadalina, I.D.; Ivanova, L.G.; Ilnitskaya, A.V.; Lipkina, L.I. Handbook on Pesticides (Toxicological and Hygienic Characteristics) (Spravochnik po Pestitsidam (Toksikologo-Gigienicheskaya Kharakteristika); Rakitskiy, V.N., Ed.; Agrorus Publishing: Moscow, Russia, 2011; 960p. (In Russian) [Google Scholar]
- Netrusov, A.I.; Egorova, M.A.; Zakharchuk, L.M.; Kolotilova, N.N.; Kotova, I.B.; Semenova, E.V.; Tatarinova, N.Y.; Ugolkova, N.V.; Tsavkelova, E.A.; Bobkova, A.F.; et al. Practical Course in Microbiology (Praktikum po Mikrobiologii); Akademiya Publishing: Moscow, Russia, 2005; 608p. (In Russian) [Google Scholar]
- Sutton, D.; Fothergill, A.; Rinaldi, M. Guide to Pathogenic and Opportunistic Fungi (Opredelitel Patogennikh i Uslovno Patogennikh Gribov); Mir Publishing: Moscow, Russia, 2001; 486p. (In Russian) [Google Scholar]
- Prudnikova, S.V.; Churakov, A.A.; Ovsyankina, S.V.; Khizhnyak, S.V. Isolation and Identification of Autochthonous Pathogens of Potato Diseases Common in the Regions of Siberia. In Proceedings of the IV International Conference “Biotechnology of new materials-Environment-Quality of life”, Krasnoyarsk, Russia, 10–13 October 2021; SibFU: Krasnoyarsk, Russia, 2021; pp. 174–177. (In Russian). [Google Scholar]
- Olkhov, A.A.; Goldshtrakh, A.M.; Zaikov, G.E.; Iordansky, A.L. Morphology of thermodynamic polyurethane and polyhydroxybutyrate blends. Bull. Technol. Univ. 2015, 18, 51–54. (In Russian) [Google Scholar]
- Olkhov, A.A.; Kosenko, R.Y.; Markin, V.S.; Staroverova, O.V.; Kucherenko, E.L.; Kurnosov, A.S.; Iordansky, A.L. Biodegradation of ultrathin fiber materials based on mixtures of polyhydroxybutyrate and polylactide. Promis. Mater. 2021, 17–31. (In Russian) [Google Scholar]
- Shah, K.R. FTIR analysis of polyhydroxyalkanoates by novel Bacillus sp. AS 3-2 from soil of Kadi region, North Gujarat, India. J. Biochem. Technol. 2012, 3, 380–383. [Google Scholar]
- Ikejima, T.; Inoue, Y. Crystallization behavior and environmental biodegradability of the blend films of poly(3-hydroxybutyric acid) with chitin and chitosan. Carbohydr. Polym. 2000, 41, 351–356. [Google Scholar] [CrossRef]
- Araujo, P.L.B.; Ferreira, C.R.P.C.; Araujo, E.S. Biodegradable conductive composites of poly(3-hydroxybutyrate) and polyaniline nanofibers: Preparation, characterization and radiolytic effects. Express Polym. Lett. 2011, 5, 12–22. [Google Scholar] [CrossRef]
- National Library of Medicine. National Center for Biotechnology Information. Prothioconazole. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/6451142 (accessed on 25 May 2022).
- National Library of Medicine. National Center for Biotechnology Information. Maetalaxyl-M. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/11150163 (accessed on 25 May 2022).
- Kumar, S.; Bhanjana, G.; Sharma, A.; Sidhu, M.C.; Dilbaghi, N. Synthesis, characterization and on field evaluation of pesticide loaded sodium alginate nanoparticles. Carbohydr. Polym. 2014, 101, 1061–1067. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Cui, B.; Zhao, X.; Zhi, H.; Zeng, Z.; Wang, Y.; Sun, C.; Liu, G.; Gao, J.; Cui, H. Antagonistic effect of azoxystrobin poly (lactic acid) microspheres with controllable particle size on Colletotrichum higginsianum Sacc. Nanomaterials 2018, 8, 857. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Cao, L.; Zhao, P.; Zhou, Z.; Cao, C.; Li, F.; Huang, Q. Emulsion-based synchronous pesticide encapsulation and surface modification of mesoporous silica nanoparticles with carboxymethyl chitosan for controlled azoxystrobin release. Chem. Eng. J. 2018, 348, 244–254. [Google Scholar] [CrossRef]
- Zhang, M.; Zhu, Z.Q.; Yuan, S.; Wang, S.Y.; Yang, C.Y.; Dwivedi, P.; Si, T.; Xu, R.X. One-step microencapsulation and spraying of pesticide formulations for improved adhesion and sustained release. J. Microencapsul. 2019, 36, 649–658. [Google Scholar] [CrossRef]
- Xu, L.; Cao, L.D.; Li, F.M.; Wang, X.J.; Huang, Q.L. Utilization of chitosan-lactide copolymer nanoparticles as controlled release pesticide carrier for pyraclostrobin against Colletotrichum gossypii Southw. J. Disp. Sci. Technol. 2014, 35, 544–550. [Google Scholar] [CrossRef]
- Barrera-Méndez, F.; Miranda-Sánchez, D.; Sánchez-Rangel, D.; Bonilla-Landa, I.; Rodríguez-Haas, B.; Monribot-Villanueva, J.L.; Olivares-Romero, J.L. Propiconazole nanoencapsulation in biodegradable polymers to obtain pesticide-controlled delivery systems. J. Mex. Chem. Soc. 2019, 63, 50–60. [Google Scholar] [CrossRef]
- Sandhya; Kumar, S.; Kumar, D.; Dilbaghi, N. Preparation, characterization, and bio-efficacy evaluation of controlled release carbendazim-loaded polymeric nanoparticles. Environ. Sci. Pollut. Res. 2017, 24, 926–937. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Sharma, D.K.; Dhiman, A. Environment friendly agar and alginate-based thiram delivery system. Toxicol. Environ. Chem. 2013, 95, 567–578. [Google Scholar] [CrossRef]
- Roshani, B.; Tavanai, H.; Morshed, M.; Khajehali, J. Controlled release of thiram pesticide from poly (L-lactic acid) nanofibers. J. Text. Inst. 2017, 108, 1504–1509. [Google Scholar] [CrossRef]
- Venugopal, N.V.S.; Sainadh, N.V.S. Novel polymeric nanoformulation of mancozeb—An eco-friendly nanomaterial. Int. J. Nanosci. 2016, 15, 1650016. [Google Scholar] [CrossRef]
- Majumder, S.; Shakil, N.A.; Kumar, J.; Banerjee, T.; Sinha, P.; Singh, B.B.; Garg, P. Eco-friendly PEG-based controlled release nano-formulations of mancozeb: Synthesis and bioefficacy evaluation against phytopathogenic fungi Alternaria solani and Sclerotium rolfsii. J. Environ. Sci. Health Part B 2016, 51, 873–880. [Google Scholar] [CrossRef]
- Duellman, K.M.; Price, W.J.; Lent, M.A.; Christian, C.L.; Bertram, M.C.; Nolte, P. Fungicide seed treatment improves performance of single-drop whole and cut seed potatoes. Am. J. Pot. Res. 2021, 98, 315–327. [Google Scholar] [CrossRef]
- Smagin, A.V.; Sadovnikova, N.B.; Vasenev, V.I.; Smagina, M.V. Biodegradation of some organic materials in soils and soil constructions: Experiments, modeling and prevention. Materials 2018, 11, 1889. [Google Scholar] [CrossRef] [Green Version]
- Smagin, A.; Sadovnikova, N.; Smagina, M. Synthetic gel structures in soils for sustainable potato farming. Sci. Rep. 2019, 9, 18588. [Google Scholar] [CrossRef]
- Mergaert, J.; Anderson, C.; Wouters, A.; Swings, J. Microbial degradation of poly(3-hydroxybutyrate) and poly(3-hydroxybutyrate-co-3-hydroxyvalerate) in compost. J. Environ. Polym. Degrad. 1994, 2, 177–183. [Google Scholar] [CrossRef]
- Jendrossek, D.; Handrick, R. Microbial degradation of polyhydroxyalkanoates. Annu. Rev. Microbiol. 2002, 56, 403–432. [Google Scholar] [CrossRef]
- Jendrossek, D.; Schirmer, A.; Schlegel, H. Biodegradation of polyhydroxyalkanoic acids. Appl. Microbiol. Biotechnol. 1996, 46, 451–463. [Google Scholar] [CrossRef]
- Sridewi, N.; Bhubalan, K.; Sudesh, K. Degradation of commercially important polyhydroxyalkanoates in tropical mangrove ecosystem. Polym. Degrad. Stab. 2006, 91, 2931–2940. [Google Scholar] [CrossRef]
- Volova, T.G.; Gladyshev, M.I.; Trusova, M.Y.; Zhila, N.O. Degradation of polyhydroxyalkanoates in eutrophic reservoir. Polym. Degrad. Stab. 2007, 92, 580–586. [Google Scholar] [CrossRef]
- Volova, T.G.; Prudnikova, S.V.; Vinogradova, O.N.; Syrvacheva, D.A.; Shishatskaya, E.I. Microbial degradation of polyhydroxyalkanoates with different chemical compositions and their biodegradability. Microb. Ecol. 2017, 73, 353–367. [Google Scholar] [CrossRef]
- Boyandin, A.N.; Rudnev, V.P.; Ivonin, V.N.; Prudnikova, S.V.; Korobikhina, K.I.; Filipenko, M.L.; Volova, T.G.; Sinskey, A.J. Biodegradation of polyhydroxyalkanoate films in natural environments. Macromol. Symp. 2012, 320, 38–42. [Google Scholar] [CrossRef]
- Boyandin, A.N.; Prudnikova, S.V.; Karpov, V.A.; Ivonin, V.N.; Đỗ, N.L.; Nguyễn, T.H.; Lê, T.M.H.; Filichev, N.L.; Levin, A.L.; Filipenko, M.L.; et al. Microbial degradation of polyhydroxyalkanoates in tropical soils. Int. Biodeterior. Biodegrad. 2013, 83, 77–84. [Google Scholar] [CrossRef]
- Volova, T.G.; Boyandin, A.N.; Vasiliev, A.D.; Karpov, A.V.; Prudnikova, S.V.; Mishukova, O.V.; Boyarskikh, U.A.; Filipenko, M.L.; Rudnev, P.V.; Xuân, B.B.; et al. Biodegradation of polyhydroxyalkanoates (PHAs) in tropical coastal waters and identification of PHA-degrading bacteria. Polym. Degrad. Stab. 2010, 95, 2350–2359. [Google Scholar] [CrossRef]
- Zhila, N.O.; Prudnikova, S.V.; Zadereev, E.S.; Rogozin, D.Y. Degradation of polyhydroxyalkanoate films in brackish Lake Shira. J. Sib. Fed. Univ. Biol. 2012, 5, 210–215. (In Russian) [Google Scholar]
- Cinelli, P.; Seggiani, M.; Mallegni, N.; Gigante, V.; Lazzeri, A. Processability and degradability of PHA-based composites in terrestrial environments. Int. J. Mol. Sci. 2019, 20, 284. [Google Scholar] [CrossRef]
- Nguyen, D.B.; Rose, M.T.; Rose, T.J.; Morris, S.G.; Van Zwieten, L. Impact of glyphosate on soil microbial biomass and respiration: A meta-analysis. Soil Biol. Biochem. 2016, 92, 50–57. [Google Scholar] [CrossRef]
- Wang, C.; Wang, F.; Zhang, Q.; Liang, W. Individual and combined effects of tebuconazole and carbendazim on soil microbial activity. Eur. J. Soil Biol. 2016, 72, 6–13. [Google Scholar] [CrossRef]
- Zhang, M.; Teng, Y.; Xu, Z.; Wang, J.; Christie, P.; Luo, Y. Cumulative effects of repeated chlorothalonil application on soil microbial activity and community in contrasting soils. J. Soils Sediments 2016, 16, 1754–1763. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, W.; Zhang, Y.; Teng, Y.; Xu, Z. Effects of fungicide iprodione and nitrification inhibitor 3,4-dimethylpyrazole phosphate on soil enzyme and bacterial properties. Sci. Total Environ. 2017, 599, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Prudnikova, S.; Streltsova, N.; Volova, T. The effect of the pesticide delivery method on the microbial community of field soil. Environ. Sci. Pollut. Res. 2021, 28, 8681–8697. [Google Scholar] [CrossRef] [PubMed]











| Name of Fungicide | Structural Formula | Use |
|---|---|---|
| Difenoconazole (a triazole fungicide); purity ≥ 95.0% |  | Effective against ascomycetes, basidiomycetes, deuteromycetes, common bunt, and root rots; protects the potato from black scurf, silver scab, fusarium disease, black dot |
| Mefenoxam (a phenylamide fungicide); purity ≥ 91.0% |  | Used to control potato diseases (late blight, early blight); used alone and mixed with other active ingredients to control plant diseases |
| Prothioconazole (a triazole fungicide); purity ≥ 98.0% |  | Effective against a wide range of plant pathogens (ascomycetes, basidiomycetes, deuteromycetes, common bunt, and root rots); protects the potato from black scurf, silver scab, fusarium disease |
| Azoxystrobin (a strobilurin fungicide); purity ≥ 98.0% |  | Suppresses a broad variety of pathogens. Protects the potato from black scurf, early blight, late blight |
| Sample | Cx, % | Tmelt, °C | Tdegr, °C | Enthalpy of Melting, (J·g−1) |
|---|---|---|---|---|
| Fungicides | ||||
| Difenoconazole | 56 | 78.6 | 329 | 60.2 |
| Azoxystrobin | 66 | 118 | 315 | 86.2 |
| Mefenoxam | - | 140 | 288 | 37.0 |
| Prothioconazole | 63 | - | 236 | - |
| Materials used to construct matrix for embedding fungicides | ||||
| P(3HB) polymer | 76 | 170 | 282 | 89.1 |
| Birch wood flour | 26 | - | 220 * | - |
| Composition and properties of experimental formulations | ||||
| P(3HB)/wood flour/difenoconazole | 43 | 78 139 | 275 343 | 5.6 51.7 |
| P(3HB)/wood flour/azoxystrobin | 55 | 118 164 | 274 347 | 7.4 44.1 |
| P(3HB)/wood flour/mefenoxam | 43 | 141 168 | 275 360 | 3.6 43.9 |
| P(3HB)/wood flour/azoxystrobin + mefenoxam | 56 | 117 137 171 | 275 348 | 2.7 1.3 29.2 |
| P(3HB)/wood flour/prothioconazole | 42 | 173 | 277 | 46.4 |
| P(3HB)/wood flour | 58 | 174 | 276 348 | 57.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Volova, T.G.; Kiselev, E.G.; Baranovskiy, S.V.; Zhila, N.O.; Prudnikova, S.V.; Shishatskaya, E.I.; Kuzmin, A.P.; Nemtsev, I.V.; Vasiliev, A.D.; Thomas, S. Degradable Poly(3-hydroxybutyrate)—The Basis of Slow-Release Fungicide Formulations for Suppressing Potato Pathogens. Polymers 2022, 14, 3669. https://doi.org/10.3390/polym14173669
Volova TG, Kiselev EG, Baranovskiy SV, Zhila NO, Prudnikova SV, Shishatskaya EI, Kuzmin AP, Nemtsev IV, Vasiliev AD, Thomas S. Degradable Poly(3-hydroxybutyrate)—The Basis of Slow-Release Fungicide Formulations for Suppressing Potato Pathogens. Polymers. 2022; 14(17):3669. https://doi.org/10.3390/polym14173669
Chicago/Turabian StyleVolova, Tatiana G., Evgeniy G. Kiselev, Sergey V. Baranovskiy, Natalia O. Zhila, Svetlana V. Prudnikova, Ekaterina I. Shishatskaya, Andrey P. Kuzmin, Ivan V. Nemtsev, Aleksander D. Vasiliev, and Sabu Thomas. 2022. "Degradable Poly(3-hydroxybutyrate)—The Basis of Slow-Release Fungicide Formulations for Suppressing Potato Pathogens" Polymers 14, no. 17: 3669. https://doi.org/10.3390/polym14173669