An Ultra-Stretchable Polyvinyl Alcohol Hydrogel Based on Tannic Acid Modified Aramid Nanofibers for Use as a Strain Sensor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of PVA/TA@ANFs/Ag Hydrogel
2.3. Structural Characterization
2.4. Properties Measurements
3. Results
3.1. Tea Stain-Inspired, Chemistry-Based ANFs
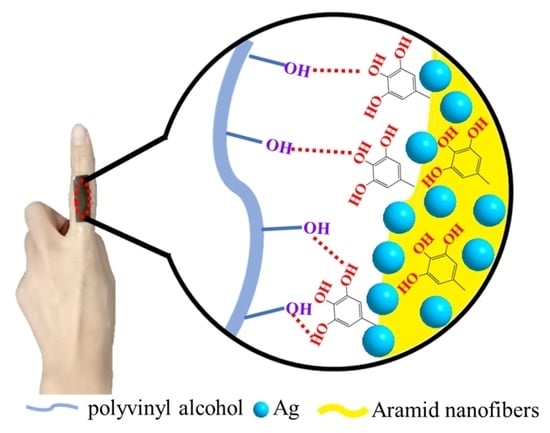
3.2. Preparation of the PVA/TA@ANFs/Ag Hydrogel
3.3. Mechanical Properties of the PVA/TA@ANFs/Ag Hydrogels
3.4. Performance of the PVA/TA@ANFs/Ag Hydrogels as Wearable Sensors
3.5. Water Absorption and Dewatering Performance of PVA/TA@ANFs/Ag Hydrogels
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, X.; Yao, F.; Li, J. Nanocomposite hydrogel-based strain and pressure sensors: A review. J. Mater. Chem. A 2020, 8, 18605–18623. [Google Scholar] [CrossRef]
- Seyedin, S.; Zhang, P.; Naebe, M.; Qin, S.; Chen, J.; Wang, X.; Razal, J.M. Textile strain sensors: A review of the fabrication technologies, performance evaluation and applications. Mater. Horiz. 2019, 6, 219–249. [Google Scholar] [CrossRef]
- Zhang, D.; Tang, Y.; Zhang, Y.; Yang, F.; Liu, Y.; Wang, X.; Yang, J.; Gong, X.; Zheng, J. Highly stretchable, self-adhesive, biocompatible, conductive hydrogels as fully polymeric strain sensors. J. Mater. Chem. A 2020, 8, 20474–20485. [Google Scholar] [CrossRef]
- Du, Y.; Yu, G.; Dai, X.; Wang, X.; Yao, B.; Kong, J. Highly Stretchable, Self-Healable, Ultrasensitive Strain and Proximity Sensors Based on Skin-Inspired Conductive Film for Human Motion Monitoring. ACS Appl. Mater. Interfaces 2020, 12, 51987–51998. [Google Scholar] [CrossRef]
- Liu, T.; Peng, X.; Chen, Y.; Zhang, J.; Jiao, C.; Wang, H. Solid-phase esterification between poly(vinyl alcohol) and malonic acid and its function in toughening hydrogels. Polym. Chem. 2020, 11, 4787–4797. [Google Scholar] [CrossRef]
- Cui, L.; Tong, W.; Zhou, H.; Yan, C.; Chen, J.; Xiong, D. PVA-BA/PEG hydrogel with bilayer structure for biomimetic articular cartilage and investigation of its biotribological and mechanical properties. J. Mater. Sci. 2020, 56, 3935–3946. [Google Scholar] [CrossRef]
- Sun, X.; Luo, C.; Luo, F. Preparation and properties of self-healable and conductive PVA-agar hydrogel with ultra-high mechanical strength. Eur. Polym. J. 2020, 124, 109465. [Google Scholar] [CrossRef]
- Du, Z.; Liu, F.; Xiao, C.; Dan, Y.; Jiang, L. Fabrication of poly(vinyl alcohol)/sodium alginate hydrogel beads and its application in photo-Fenton degradation of tetracycline. J. Mater. Sci. 2020, 56, 913–926. [Google Scholar] [CrossRef]
- Zhang, Y.-F.; Guo, M.-M.; Zhang, Y.; Tang, C.Y.; Jiang, C.; Dong, Y.; Law, W.-C.; Du, F.-P. Flexible, stretchable and conductive PVA/PEDOT:PSS composite hydrogels prepared by SIPN strategy. Polym. Test. 2020, 81, 106213. [Google Scholar] [CrossRef]
- Liang, Y.; Sun, X.; Lv, Q.; Shen, Y.; Liang, H. Fully physically cross-linked hydrogel as highly stretchable, tough, self-healing and sensitive strain sensors. Polymer 2020, 210, 123039. [Google Scholar] [CrossRef]
- Ye, F.; Li, M.; Ke, D.; Wang, L.; Lu, Y. Ultrafast Self-Healing and Injectable Conductive Hydrogel for Strain and Pressure Sensors. Adv. Mater. Technol. 2019, 4, 1900346. [Google Scholar] [CrossRef]
- Zheng, W.; Li, Y.; Xu, L.; Huang, Y.; Jiang, Z.; Li, B. Highly stretchable, healable, sensitive double-network conductive hydrogel for wearable sensor. Polymer 2020, 211, 123095. [Google Scholar] [CrossRef]
- Jing, H.; Shi, J.; Guoab, P.; Guan, S.; Fu, H.; Cui, W. Hydrogels based on physically cross-linked network with high mechanical property and recasting ability. Colloid Surf. A 2021, 611, 125805. [Google Scholar] [CrossRef]
- Jing, X.; Li, H.; Mi, H.-Y.; Liu, Y.-J.; Feng, P.-Y.; Tan, Y.-M.; Turng, L.-S. Highly transparent, stretchable, and rapid self-healing polyvinyl alcohol/cellulose nanofibril hydrogel sensors for sensitive pressure sensing and human motion detection. Sens. Actuat. B-Chem. 2019, 295, 159–167. [Google Scholar] [CrossRef]
- Yang, N.; Qi, P.; Ren, J.; Yu, H.; Liu, S.; Li, J.; Chen, W.; Kaplan, D.L.; Ling, S. Polyvinyl Alcohol/Silk Fibroin/Borax Hydrogel Ionotronics: A Highly Stretchable, Self-Healable, and Biocompatible Sensing Platform. ACS Appl. Mater. Interfaces 2019, 11, 23632–23638. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, B.; Yao, B.; Qiu, Y.; Peng, Z.; Zhang, Y.; Alsaid, Y.; Frenkel, I.; Youssef, K.; Pei, Q.; et al. Hierarchically Structured Stretchable Conductive Hydrogels for High-Performance Wearable Strain Sensors and Supercapacitors. Matter 2020, 3, 1196–1210. [Google Scholar] [CrossRef]
- Huang, H.; Yao, J.; Li, L.; Zhu, F.; Liu, Z.; Zeng, X.; Yu, X.; Huang, Z. Reinforced polyaniline/polyvinyl alcohol conducting hydrogel from a freezing–thawing method as self-supported electrode for supercapacitors. J. Mater. Sci. 2016, 51, 8728–8736. [Google Scholar] [CrossRef]
- Lin, J.X.; Hu, H.W.; Luo, J.; Miao, L.; Yang, Z.H.; Chen, M.; Zhang, M.; Ou, J.Z. Micro/nanoarrays and their applications in flexible sensors: A review. Mater. Today Nano 2022, 19, 100224. [Google Scholar] [CrossRef]
- Azadi, S.; Peng, S.; Moshizi, S.A.; Asadnia, M.; Xu, J.; Park, I.; Wang, C.H.; Wu, S. Biocompatible and Highly Stretchable PVA/AgNWs Hydrogel Strain Sensors for Human Motion Detection. Adv. Mater. Technol. 2020, 5, 2000426. [Google Scholar] [CrossRef]
- Cheng, B.; Chang, S.; Li, H.; Li, Y.; Shen, W.; Shang, Y.; Cao, A. Highly Stretchable and Compressible Carbon Nanofiber–Polymer Hydrogel Strain Sensor for Human Motion Detection. Macromol. Mater. Eng. 2020, 305, 1900813. [Google Scholar] [CrossRef]
- Lin, F.; Wang, Z.; Shen, Y.; Tang, L.; Zhang, P.; Wang, Y.; Chen, Y.; Huang, B.; Lu, B. Natural skin-inspired versatile cellulose biomimetic hydrogels. J. Mater. Chem. A 2019, 7, 26442–26455. [Google Scholar] [CrossRef]
- Wang, J.; Lin, Y.; Mohamed, A.; Ji, Q.; Jia, H. High strength and flexible aramid nanofiber conductive hydrogels for wearable strain sensors. J. Mater. Chem. C 2021, 9, 575–583. [Google Scholar] [CrossRef]
- Yang, B.; Wang, L.; Zhang, M.; Luo, J.; Ding, X. Timesaving, High-Efficiency Approaches to Fabricate Aramid Nanofibers. ACS Nano 2019, 13, 7886–7897. [Google Scholar] [CrossRef]
- Tung, S.; Fisher, S.L.; Kotov, N.A.; Thompson, L.T. Nanoporous aramid nanofibre separators for nonaqueous redox flow batteries. Nat. Commun. 2018, 9, 4193. [Google Scholar] [CrossRef] [PubMed]
- Miao, L.; Wu, Y.; Hu, J.; Wang, P.; Liu, G.; Lin, S.; Tu, Y. Hierarchical aramid nanofibrous membranes from a nanofiber-based solvent-induced phase inversion process. J. Membr. Sci. 2019, 578, 16–26. [Google Scholar] [CrossRef]
- Miao, L.; Jiang, T.; Lin, S.; Jin, T.; Hu, J.; Zhang, M.; Tu, Y.; Liu, G. Asymmetric forward osmosis membranes from p-aramid nanofibers. Mater. Des. 2020, 191, 108591. [Google Scholar] [CrossRef]
- Xu, L.; Zhao, X.; Xu, C.; Kotov, N.A. Water-Rich Biomimetic Composites with Abiotic Self-Organizing Nanofiber Network. Adv. Mater. 2018, 30, 1703343. [Google Scholar] [CrossRef]
- Du, W.; Zhang, J.; Zhao, Z.; Zhang, X. Preparation of novel temperature-responsive double-network hydrogel reinforced with aramid nanofibers. Compos. Commun. 2020, 22, 100438. [Google Scholar] [CrossRef]
- Chen, Y.-N.; Peng, L.; Liu, T.; Wang, Y.; Shi, S.; Wang, H. Poly(vinyl alcohol)–Tannic Acid Hydrogels with Excellent Mechanical Properties and Shape Memory Behaviors. ACS Appl. Mater. Interfaces 2016, 8, 27199–27206. [Google Scholar] [CrossRef]
- Ari, B.; Sahiner, M.; Demirci, S.; Sahiner, N. Poly(vinyl alcohol)-tannic Acid Cryogel Matrix as Antioxidant and Antibacterial Material. Polymers 2022, 14, 70. [Google Scholar] [CrossRef]
- Sathishkumar, G.; Gopinath, K.; Zhang, K.; Kang, E.T.; Xu, L.; Yu, Y. Recent progress in tannic acid-driven antibacterial/antifouling surface coating strategies. J. Mater. Chem. B 2022, 10, 2296–2315. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Zhou, Z.; Xie, A.; Meng, M.; Cui, Y.; Liu, S.; Lu, J.; Zhou, S.; Yan, Y.; Dong, H. Bio-inspired fabrication of superhydrophilic nanocomposite membrane based on surface modification of SiO2 anchored by polydopamine towards effective oil-water emulsions separation. Sep. Purif. Technol. 2019, 209, 434–442. [Google Scholar] [CrossRef]
- Peng, M.; Xiao, G.; Tang, X.; Zhou, Y. Hydrogen-Bonding Assembly of Rigid-Rod Poly(p-sulfophenylene terephthalamide) and Flexible-Chain Poly(vinyl alcohol) for Transparent, Strong, and Tough Molecular Composites. Macromolecules 2014, 47, 8411–8419. [Google Scholar] [CrossRef]
- Guan, Y.; Li, W.; Zhang, Y.; Shi, Z.; Tan, J.; Wang, F.; Wang, Y. Aramid nanofibers and poly (vinyl alcohol) nanocomposites for ideal combination of strength and toughness via hydrogen bonding interactions. Compos. Sci. Technol. 2017, 144, 193–201. [Google Scholar] [CrossRef]
- Yan, W.; Shi, M.; Dong, C.; Liu, L.; Gao, C. Applications of tannic acid in membrane technologies: A review. Adv. Colloid Interfaces Sci. 2020, 284, 102267. [Google Scholar] [CrossRef]
- Mather, R.R. Surface modification of textiles by plasma treatments. In Surface Modification of Textiles; Wei, Q., Ed.; Woodhead Publishing: Sawston Cambridge, UK, 2009; pp. 296–317. [Google Scholar]
- Peppas, N.A.; Hansen, P.J. Crystallization kinetics of poly(vinyl alcohol). J. Appl. Polym. Sci. 1982, 27, 4787–4797. [Google Scholar] [CrossRef]
- Ohtaki, H. Effects of temperature and pressure on hydrogen bonds in water and in formamide. J. Mol. Liq. 2003, 103–104, 3–13. [Google Scholar] [CrossRef]
- Holloway, J.L.; Lowman, A.M.; Palmese, G.R. The role of crystallization and phase separation in the formation of physically cross-linked PVA hydrogels. Soft Matter 2013, 9, 826–833. [Google Scholar] [CrossRef]
- Guan, Y.; Shao, L.; Dong, D.; Wang, F.; Zhang, Y.; Wang, Y. Bio-inspired natural polyphenol cross-linking poly(vinyl alcohol) films with strong integrated strength and toughness. RSC Adv. 2016, 6, 69966–69972. [Google Scholar] [CrossRef]
- Díaz-Cruz, C.; Alonso Nuñez, G.; Espinoza-Gómez, H.; Flores-López, L.Z. Effect of molecular weight of PEG or PVA as reducing-stabilizing agent in the green synthesis of silver-nanoparticles. Eur. Polym. J. 2016, 83, 265–277. [Google Scholar] [CrossRef]
- Kaabipour, S.; Hemmati, S. A review on the green and sustainable synthesis of silver nanoparticles and one-dimensional silver nanostructures. Beilstein J. Nanotechnol. 2021, 12, 102–136. [Google Scholar]
- Lopes, L.C.S.; Brito, L.M.; Bezerra, T.T.; Gomes, K.N.; Carvalho, F.A.D.A.; Chaves, M.H.; Cantanhêde, W. Silver and gold nanoparticles from tannic acid: Synthesis, characterization and evaluation of antileishmanial and cytotoxic activities. An. Acad. Bras. Cienc. 2018, 90, 2679–2689. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Hu, K.; Zhao, C.; Zou, Y.; Liu, Y.; Qu, X.; Jiang, D.; Li, Z.; Zhang, M.R.; Li, Z. Customization of Conductive Elastomer Based on PVA/PEI for Stretchable Sensors. Small 2020, 16, e1904758. [Google Scholar] [CrossRef] [PubMed]
- Steiner, T. The Hydrogen Bond in the Solid State. Angew. Chem. Int. Edit. 2002, 41, 48–76. [Google Scholar] [CrossRef]
- Chen, Y.; Jiao, C.; Peng, X.; Liu, T.; Shi, Y.; Liang, M.; Wang, H. Biomimetic anisotropic poly(vinyl alcohol) hydrogels with significantly enhanced mechanical properties by freezing–thawing under drawing. J. Mater. Chem. B 2019, 7, 3243–3249. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, F.; Yazigi, S.; Zhang, D.; Gui, X.; Qi, Y.; Zhong, J.; Sun, L. Nanoengineered highly sensitive and stable soft strain sensor built from cracked carbon nanotube network/composite bilayers. Carbon 2021, 173, 849–856. [Google Scholar] [CrossRef]
- Wu, X.; Li, W.; Chen, K.; Zhang, D.; Xu, L.; Yang, X. A tough PVA/HA/COL composite hydrogel with simple process and excellent mechanical properties. Mater. Today Commun. 2019, 21, 100702. [Google Scholar] [CrossRef]
- Luo, X.; Akram, M.Y.; Yuan, Y.; Nie, J.; Zhu, X. Silicon dioxide/poly(vinyl alcohol) composite hydrogels with high mechanical properties and low swellability. J. Appl. Polym. Sci. 2019, 136, 46895. [Google Scholar] [CrossRef] [Green Version]









| Elemental Compositions | C (%) | N (%) | O (%) |
|---|---|---|---|
| Neat ANFs | 72.07 | 17.77 | 10.16 |
| TA@ANFs | 73.80 | 13.87 | 12.33 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miao, L.; Wang, X.; Li, S.; Tu, Y.; Hu, J.; Huang, Z.; Lin, S.; Gui, X. An Ultra-Stretchable Polyvinyl Alcohol Hydrogel Based on Tannic Acid Modified Aramid Nanofibers for Use as a Strain Sensor. Polymers 2022, 14, 3532. https://doi.org/10.3390/polym14173532
Miao L, Wang X, Li S, Tu Y, Hu J, Huang Z, Lin S, Gui X. An Ultra-Stretchable Polyvinyl Alcohol Hydrogel Based on Tannic Acid Modified Aramid Nanofibers for Use as a Strain Sensor. Polymers. 2022; 14(17):3532. https://doi.org/10.3390/polym14173532
Chicago/Turabian StyleMiao, Lei, Xiao Wang, Shi Li, Yuanyuan Tu, Jiwen Hu, Zhenzhu Huang, Shudong Lin, and Xuefeng Gui. 2022. "An Ultra-Stretchable Polyvinyl Alcohol Hydrogel Based on Tannic Acid Modified Aramid Nanofibers for Use as a Strain Sensor" Polymers 14, no. 17: 3532. https://doi.org/10.3390/polym14173532