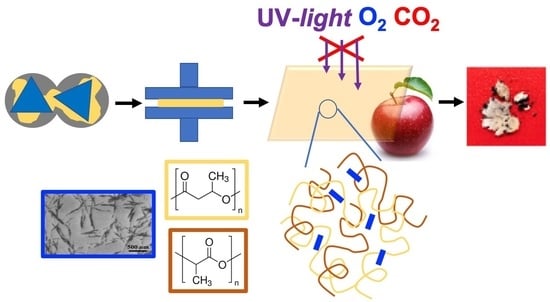
Chitin Nanocomposite Based on Plasticized Poly(lactic acid)/Poly(3-hydroxybutyrate) (PLA/PHB) Blends as Fully Biodegradable Packaging Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Chitin Nanoparticles
2.3. Film Preparation
2.4. Microscopy
2.5. Infrared Spectroscopy
2.6. Wide Angle X-ray Scattering
2.7. Water Contact Angle Measurements
2.8. Thermal Analysis
2.9. Optical and Colorimetric Properties
2.10. Mechanical Properties
2.11. Barrier Properties
2.12. Overall Migration in Food Simulants
2.13. Disintegration under Composting Conditions
3. Results and Discussion
3.1. Chitin Nanoparticles
3.2. PLA/PHB Bio-Nanocomposite
3.2.1. Morphological and Structural Characterization
3.2.2. Water Contact Angle
3.2.3. Optical and Colorimetric Properties
3.2.4. Thermal Characterization
3.2.5. Mechanical Characterization
3.2.6. Barrier Properties
3.2.7. Overall Migration
3.2.8. Disintegration under Composting Conditions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, J.; Euring, M.; Ostendorf, K.; Zhang, K. Biobased materials for food packaging. J. Bioresour. Bioprod. 2022, 7, 1–13. [Google Scholar] [CrossRef]
- Burgos, N.; Armentano, I.; Fortunati, E.; Dominici, F.; Luzi, F.; Fiori, S.; Cristofaro, F.; Visai, L.; Jiménez, A.; Kenny, J.M. Functional properties of plasticized bio-based poly(lactic acid)_poly(hydroxybutyrate) (PLA_PHB) films for active food packaging. Food Bioprocess Technol. 2017, 10, 770–780. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Ma, P.; Wang, R.; Wang, S.; Zhang, Y.; Zhang, Y. Mechanical, thermal and degradation properties of poly(d,l-lactide)/poly(hydroxybutyrate-co-hydroxyvalerate)/poly(ethylene glycol) blend. Polym. Degrad. Stab. 2008, 93, 1364–1369. [Google Scholar] [CrossRef]
- Arrieta, M.P.; López, J.; Hernández, A.; Rayón, E. Ternary PLA–PHB–Limonene blends intended for biodegradable food packaging applications. Eur. Polym. J. 2014, 50, 255–270. [Google Scholar] [CrossRef]
- D’Amico, D.A.; Montes, M.L.I.; Manfredi, L.B.; Cyras, V.P. Fully bio-based and biodegradable polylactic acid/poly(3-hydroxybutirate) blends: Use of a common plasticizer as performance improvement strategy. Polym. Test. 2016, 49, 22–28. [Google Scholar] [CrossRef]
- Abdelwahab, M.A.; Flynn, A.; Chiou, B.-S.; Imam, S.; Orts, W.; Chiellini, E. Thermal, mechanical and morphological characterization of plasticized PLA–PHB blends. Polym. Degrad. Stab. 2012, 97, 1822–1828. [Google Scholar] [CrossRef]
- Arora, A.; Padua, G.W. Review: Nanocomposites in Food Packaging. J. Food Sci. 2010, 75, R43–R49. [Google Scholar] [CrossRef] [PubMed]
- Arrieta, M.P.; Peponi, L.; López, D.; López, J.; Kenny, J.M. An overview of nanoparticles role in the improvement of barrier properties of bioplastics for food packaging applications. In Food Packaging; Elsevier: Amsterdam, The Netherlands, 2017; pp. 391–424. ISBN 9780128043028. [Google Scholar]
- Raquez, J.-M.; Habibi, Y.; Murariu, M.; Dubois, P. Polylactide (PLA)-based nanocomposites. Prog. Polym. Sci. 2013, 38, 1504–1542. [Google Scholar] [CrossRef]
- Zhao, L.; Duan, G.; Zhang, G.; Yang, H.; He, S.; Jiang, S. Electrospun functional materials toward food packaging applications: A review. Nanomaterials 2020, 10, 150. [Google Scholar] [CrossRef] [Green Version]
- Plucinski, A.; Lyu, Z.; Schmidt, B.V.K.J. Polysaccharide nanoparticles: From fabrication to applications. J. Mater. Chem. B 2021, 9, 7030–7062. [Google Scholar] [CrossRef] [PubMed]
- Chivrac, F.; Pollet, E.; Avérous, L. Progress in nano-biocomposites based on polysaccharides and nanoclays. Mater. Sci. Eng. R Rep. 2009, 67, 1–17. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Fortunati, E.; Dominici, F.; Rayón, E.; López, J.; Kenny, J.M. Multifunctional PLA-PHB/cellulose nanocrystal films: Processing, structural and thermal properties. Carbohydr. Polym. 2014, 107, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Arrieta, M.P.; Fortunati, E.; Dominici, F.; Rayón, E.; López, J.; Kenny, J.M. PLA-PHB/cellulose based films: Mechanical, barrier and disintegration properties. Polym. Degrad. Stab. 2014, 107, 139–149. [Google Scholar] [CrossRef]
- Arrieta, M.P.; López, J.; López, D.; Kenny, J.M.; Peponi, L. Biodegradable electrospun bionanocomposite fibers based on plasticized PLA–PHB blends reinforced with cellulose nanocrystals. Ind. Crop. Prod. 2016, 93, 290–301. [Google Scholar] [CrossRef]
- Kiziltas, A.; Nazari, B.; Erbas Kiziltas, E.; Gardner, D.J.; Han, Y.; Rushing, T.S. Method to reinforce polylactic acid with cellulose nanofibers via a polyhydroxybutyrate carrier system. Carbohydr. Polym. 2016, 140, 393–399. [Google Scholar] [CrossRef]
- Kim, S.-K. Chitin, Chitosan, Oligosaccharides and Their Derivatives. Biological Activities and Applications; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: Oxfordshire, UK, 2011. [Google Scholar]
- Peniche, C.; Argüelles-Monal, W.; Goycoolea, F.M. Chitin and chitosan: Major sources, properties and applications. In Monomers, Polymers and Composites from Renewable Resources; Elsevier: Amsterdam, The Netherlands, 2008; Volume 1, pp. 517–542. ISBN 9780080453163. [Google Scholar]
- Coltelli, M.-B.; Cinelli, P.; Gigante, V.; Aliotta, L.; Morganti, P.; Panariello, L.; Lazzeri, A. Chitin nanofibrils in poly(lactic acid) (PLA) nanocomposites: Dispersion and thermo-mechanical properties. Int. J. Mol. Sci. 2019, 20, 504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salaberria, A.M.; Labidi, J.; Fernandes, S.C.M.M. Different routes to turn chitin into stunning nano-objects. Eur. Polym. J. 2015, 68, 503–515. [Google Scholar] [CrossRef]
- Paillet, M.; Dufresne, A. Chitin whisker reinforced thermoplastic nanocomposites. Macromolecules 2001, 34, 6527–6530. [Google Scholar] [CrossRef]
- Oun, A.A.; Rhim, J.-W. Preparation of multifunctional chitin nanowhiskers/ZnO-Ag NPs and their effect on the properties of carboxymethyl cellulose-based nanocomposite film. Carbohydr. Polym. 2017, 169, 467–479. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.R.; Jian, R.; Yu, J.; Ma, X. Starch-based composites reinforced with novel chitin nanoparticles. Carbohydr. Polym. 2010, 80, 420–425. [Google Scholar] [CrossRef]
- Salaberria, A.M.; Diaz, R.H.; Labidi, J.; Fernandes, S.C.M.M. Role of chitin nanocrystals and nanofibers on physical, mechanical and functional properties in thermoplastic starch films. Food Hydrocoll. 2015, 46, 93–102. [Google Scholar] [CrossRef]
- Araki, J.; Yamanaka, Y.; Ohkawa, K. Chitin-chitosan nanocomposite gels: Reinforcement of chitosan hydrogels with rod-like chitin nanowhiskers. Polym. J. 2012, 44, 713–717. [Google Scholar] [CrossRef] [Green Version]
- Deng, Q.; Li, J.; Yang, J.; Li, D. Optical and flexible α-chitin nanofibers reinforced poly(vinyl alcohol) (PVA) composite film: Fabrication and property. Compos. Part A Appl. Sci. Manuf. 2014, 67, 55–60. [Google Scholar] [CrossRef]
- Herrera, N.; Roch, H.; Salaberria, A.M.; Pino-Orellana, M.A.; Labidi, J.; Fernandes, S.C.M.; Radic, D.; Leiva, A.; Oksman, K. Functionalized blown films of plasticized polylactic acid/chitin nanocomposite: Preparation and characterization. Mater. Des. 2016, 92, 846–852. [Google Scholar] [CrossRef]
- Herrera, N.; Salaberria, A.M.; Mathew, A.P.; Oksman, K. Plasticized polylactic acid nanocomposite films with cellulose and chitin nanocrystals prepared using extrusion and compression molding with two cooling rates: Effects on mechanical, thermal and optical properties. Compos. Part A Appl. Sci. Manuf. 2016, 83, 89–97. [Google Scholar] [CrossRef] [Green Version]
- Meng, D.; Xie, J.; Waterhouse, G.I.N.; Zhang, K.; Zhao, Q.; Wang, S.; Qiu, S.; Chen, K.; Li, J.; Ma, C.; et al. Biodegradable poly(butylene adipate-co-terephthalate) composites reinforced with bio-based nanochitin: Preparation, enhanced mechanical and thermal properties. J. Appl. Polym. Sci. 2020, 137, 48485. [Google Scholar] [CrossRef]
- Oyeoka, H.C.; Ewulonu, C.M.; Nwuzor, I.C.; Obele, C.M.; Nwabanne, J.T. Packaging and degradability properties of polyvinyl alcohol/gelatin nanocomposite films filled water hyacinth cellulose nanocrystals. J. Bioresour. Bioprod. 2021, 6, 168–185. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, W.-R.; Zhang, Y.-C.; Han, X.-D.; Chen, C.; Chen, A. In situ generated silica reinforced polyvinyl alcohol/liquefied chitin biodegradable films for food packaging. Carbohydr. Polym. 2020, 238, 116182. [Google Scholar] [CrossRef] [PubMed]
- Guan, Q.; Naguib, H.E. Fabrication and characterization of PLA/PHBV-chitin nanocomposites and their foams. J. Polym. Environ. 2014, 22, 119–130. [Google Scholar] [CrossRef]
- Iglesias-Montes, M.L.; D’amico, D.A.; Manfredi, L.B.; Cyras, V.P. Effect of natural glyceryl tributyrate as plasticizer and compatibilizer on the performance of bio-based polylactic acid/poly(3-hydroxybutyrate) blends. J. Polym. Environ. 2019, 27, 1429–1438. [Google Scholar] [CrossRef]
- Iglesias-Montes, M.L.; Cyras, V.P.; Manfredi, L.B.; Pettarín, V.; Fasce, L.A. Fracture evaluation of plasticized polylactic acid/poly (3-hydroxybutyrate) blends for commodities replacement in packaging applications. Polym. Test. 2020, 84, 106375. [Google Scholar] [CrossRef]
- Iglesias-Montes, M.L.; Soccio, M.; Luzi, F.; Puglia, D.; Gazzano, M.; Lotti, N.; Manfredi, L.B.; Cyras, V.P. Evaluation of the factors affecting the disintegration under a composting process of poly(lactic acid)/poly(3-hydroxybutyrate) (PLA/PHB) blends. Polymers 2021, 13, 3171. [Google Scholar] [CrossRef] [PubMed]
- Nair, K.G.; Dufresne, A. Crab shell chitin whisker reinforced natural rubber nanocomposites. 1. Processing and swelling behavior. Biomacromolecules 2003, 4, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Goodrich, J.D.; Winter, W.T. α-chitin nanocrystals prepared from shrimp shells and their specific surface area measurement. Biomacromolecules 2007, 8, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Barham, P.J.; Keller, A.; Otun, E.L.; Holmes, P.A. Crystallization and morphology of a bacterial thermoplastic: Poly-3-hydroxybutyrate. J. Mater. Sci. 1984, 19, 2781–2794. [Google Scholar] [CrossRef]
- Avérous, L. Polylactic acid: Synthesis, properties and applications. In Monomers, Polymers and Composites from Renewable Resources; Belgacem, N., Gandini, A., Eds.; Elsevier Limited Publication: Londres, UK, 2008; pp. 433–450. ISBN 9780080453163. [Google Scholar]
- European, C. Commission Regulation (EU) No 10/2011 of 14 January 2011 on plastic materials and articles intended to come into contact with food. Off. J. Eur. Union 2011, 12L, 1–89. [Google Scholar]
- Phongying, S.; Aiba, S.; Chirachanchai, S. Direct chitosan nanoscaffold formation via chitin whiskers. Polymer 2007, 48, 393–400. [Google Scholar] [CrossRef]
- Rubentheren, V.; Ward, T.A.; Chee, C.Y.; Tang, C.K. Processing and analysis of chitosan nanocomposites reinforced with chitin whiskers and tannic acid as a crosslinker. Carbohydr. Polym. 2015, 115, 379–387. [Google Scholar] [CrossRef]
- Dahmane, E.M.; Taourirte, M.; Eladlani, N.; Rhazi, M. Extraction and characterization of chitin and chitosan from parapenaeus longirostris from Moroccan local sources. Int. J. Polym. Anal. Charact. 2014, 19, 342–351. [Google Scholar] [CrossRef]
- Kadokawa, J.; Takegawa, A.; Mine, S.; Prasad, K. Preparation of chitin nanowhiskers using an ionic liquid and their composite materials with poly(vinyl alcohol). Carbohydr. Polym. 2011, 84, 1408–1412. [Google Scholar] [CrossRef]
- Xie, Y.; Hill, C.A.S.; Xiao, Z.; Militz, H.; Mai, C. Silane coupling agents used for natural fiber/polymer composites: A review. Compos. Part A Appl. Sci. Manuf. 2010, 41, 806–819. [Google Scholar] [CrossRef]
- Furukawa, T.; Sato, H.; Murakami, R.; Zhang, J.; Duan, Y.X.; Noda, I.; Ochiai, S.; Ozaki, Y. Structure, dispersibility and crystallinity of poly (hydroxybutyrate)/poly(l-lactic acid) blends studied by FT-IR microspectroscopy and differential scanning calorimetry. Macromolecules 2005, 38, 6445–6454. [Google Scholar] [CrossRef]
- Oliveira, L.M.; Araújo, E.S.; Guedes, S.M.L. Gamma irradiation effects on poly(hydroxybutyrate). Polym. Degrad. Stab. 2006, 91, 2157–2162. [Google Scholar] [CrossRef]
- Zhang, M.; Thomas, N.L. Blending polylactic acid with polyhydroxybutyrate: The effect on thermal, mechanical, and biodegradation properties. Adv. Polym. Technol. 2011, 30, 67–79. [Google Scholar] [CrossRef]
- Vogler, E.A. Structure and reactivity of water at biomaterial surfaces. Adv. Colloid Interface Sci. 1998, 74, 69–117. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Samper, M.D.; López, J.; Jiménez, A. Combined effect of poly(hydroxybutyrate) and plasticizers on polylactic acid properties for film intended for food packaging. J. Polym. Environ. 2014, 22, 460–470. [Google Scholar] [CrossRef]
- Bradley, R.L. Effect of light on alteration of nutritional value and flavor of milk: A review. J. Food Prot. 1980, 43, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Auras, R.; Harte, B.; Selke, S. An overview of polylactides as packaging materials. Macromol. Biosci. 2004, 4, 835–864. [Google Scholar] [CrossRef]
- Garcia-Garcia, D.; Lopez-Martinez, J.; Balart, R.; Strömberg, E.; Moriana, R. Reinforcing capability of cellulose nanocrystals obtained from pine cones in a biodegradable poly(3-hydroxybutyrate)/poly(ε-caprolactone) (PHB/PCL) thermoplastic blend. Eur. Polym. J. 2018, 104, 10–18. [Google Scholar] [CrossRef]
- Sharma, G. Digital Color Imaging Handbook; Sharma, G., Ed.; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: New York, NY, USA, 2003. [Google Scholar]
- Rodriguez-Gonzalez, F.; Ramsay, B.; Favis, B. High performance LDPE/thermoplastic starch blends: A sustainable alternative to pure polyethylene. Polymer 2003, 44, 1517–1526. [Google Scholar] [CrossRef]
- Li, J.-H.; Miao, J.; Wu, J.-L.; Chen, S.-F.; Zhang, Q.-Q. Preparation and characterization of active gelatin-based films incorporated with natural antioxidants. Food Hydrocoll. 2014, 37, 166–173. [Google Scholar] [CrossRef]
- Lemmouchi, Y.; Murariu, M.; Santos, A.M.D.; Amass, A.J.; Schacht, E.; Dubois, P. Plasticization of poly(lactide) with blends of tributyl citrate and low molecular weight poly(d,l-lactide)-b-poly(ethylene glycol) copolymers. Eur. Polym. J. 2009, 45, 2839–2848. [Google Scholar] [CrossRef]
- Murariu, M.; Ferreira, A.D.S.; Alexandre, M.; Dubois, P. Polylactide (PLA) designed with desired end-use properties: 1. PLA compositions with low molecular weight ester-like plasticizers and related performances. Polym. Adv. Technol. 2008, 19, 636–646. [Google Scholar] [CrossRef]
- Ljungberg, N.; Wesslén, B. Tributyl citrate oligomers as plasticizers for poly (lactic acid): Thermo-mechanical film properties and aging. Polymer 2003, 44, 7679–7688. [Google Scholar] [CrossRef]
- Burgos, N.; Martino, V.P.; Jiménez, A. Characterization and ageing study of poly(lactic acid) films plasticized with oligomeric lactic acid. Polym. Degrad. Stab. 2013, 98, 651–658. [Google Scholar] [CrossRef]
- Zhang, J.; Tashiro, K.; Tsuji, H.; Domb, A.J. Disorder-to-order phase transition and multiple melting behavior of poly(L-lactide) investigated by simultaneous measurements of WAXD and DSC. Macromolecules 2008, 41, 1352–1357. [Google Scholar] [CrossRef]
- Râpă, M.; Darie-Nită, R.N.; Grosu, E.; Tănase, E.E.; Trifoi, A.R.; Pap, T.; Vasile, C. Effect of plasticizers on melt processability and properties of PHB. J. Optoelectron. Adv. Mater. 2015, 17, 1778–1784. [Google Scholar]
- Erceg, M.; Kovačić, T.; Klarić, I. Thermal degradation of poly(3-hydroxybutyrate) plasticized with acetyl tributyl citrate. Polym. Degrad. Stab. 2005, 90, 313–318. [Google Scholar] [CrossRef]
- Tri, P.N.; Domenek, S.; Guinault, A.; Sollogoub, C. Crystallization behavior of poly(lactide)/poly(β-hydroxybutyrate)/talc composites. J. Appl. Polym. Sci. 2013, 129, 3355–3365. [Google Scholar] [CrossRef] [Green Version]
- Groenewoud, W.M. Characterisation of Polymers by Thermal Analysis; Elsevier Science: Amsterdam, The Netherlands, 2001; pp. 10–60. [Google Scholar]
- Nanda, M.R.; Misra, M.; Mohanty, A.K. The effects of process engineering on the performance of PLA and PHBV blends. Macromol. Mater. Eng. 2011, 296, 719–728. [Google Scholar] [CrossRef]
- Bartczak, Z.; Galeski, A.; Kowalczuk, M.; Sobota, M.; Malinowski, R. Tough blends of poly(lactide) and amorphous poly([R,S]-3-hydroxy butyrate)—Morphology and properties. Eur. Polym. J. 2013, 49, 3630–3641. [Google Scholar] [CrossRef]
- Ma, P.; Spoelstra, A.B.; Schmit, P.; Lemstra, P.J. Toughening of poly (lactic acid) by poly (β-hydroxybutyrate-co-β-hydroxyvalerate) with high β-hydroxyvalerate content. Eur. Polym. J. 2013, 49, 1523–1531. [Google Scholar] [CrossRef]
- Fortunati, E.; Armentano, I.; Zhou, Q.; Puglia, D.; Terenzi, A.; Berglund, L.A.; Kenny, J.M. Microstructure and nonisothermal cold crystallization of PLA composites based on silver nanoparticles and nanocrystalline cellulose. Polym. Degrad. Stab. 2012, 97, 2027–2036. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Fortunati, E.; Dominici, F.; López, J.; Kenny, J.M. Bionanocomposite films based on plasticized PLA-PHB/cellulose nanocrystal blends. Carbohydr. Polym. 2015, 121, 265–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jamshidian, M.; Tehrany, E.A.; Imran, M.; Akhtar, M.J.; Cleymand, F.; Desobry, S. Structural, mechanical and barrier properties of active PLA–antioxidant films. J. Food Eng. 2012, 110, 380–389. [Google Scholar] [CrossRef]
- Drieskens, M.; Peeters, R.; Mullens, J.; Franco, D.; Lemstra, P.J.; Hristova-Bogaerds, D.G. Structure versus properties relationship of poly(lactic acid). I. effect of crystallinity on barrier properties. J. Polym. Sci. Part B Polym. Phys. 2009, 47, 2247–2258. [Google Scholar] [CrossRef]
- Wang, Y.; Qin, Y.; Zhang, Y.; Yuan, M.; Li, H.; Yuan, M. Effects of N-octyl lactate as plasticizer on the thermal and functional properties of extruded PLA-based films. Int. J. Biol. Macromol. 2014, 67, 58–63. [Google Scholar] [CrossRef]
- Arrieta, M.P.; López, J.; Ferrándiz, S.; Peltzer, M.A. Characterization of PLA-limonene blends for food packaging applications. Polym. Test. 2013, 32, 760–768. [Google Scholar] [CrossRef]
- Kalogeras, I.M.; Brostow, W. Glass transition temperatures in binary polymer blends. J. Polym. Sci. Part B Polym. Phys. 2009, 47, 80–95. [Google Scholar] [CrossRef]
- Guidotti, G.; Soccio, M.; Siracusa, V.; Gazzano, M.; Munari, A.; Lotti, N. Novel random copolymers of poly(butylene 1,4-cyclohexane dicarboxylate) with outstanding barrier properties for green and sustainable packaging: Content and length of aliphatic side chains as efficient tools to tailor the material’s final performance. Polymers 2018, 10, 866. [Google Scholar] [CrossRef] [Green Version]
- Dhar, P.; Bhardwaj, U.; Kumar, A.; Katiyar, V. Poly(3-hydroxybutyrate)/cellulose nanocrystal films for food packaging applications: Barrier and migration studies. Polym. Eng. Sci. 2015, 55, 2388–2395. [Google Scholar] [CrossRef]
- Kale, G.; Kijchavengkul, T.; Auras, R.; Rubino, M.; Selke, S.E.; Singh, S.P. Compostability of bioplastic packaging materials: An overview. Macromol. Biosci. 2007, 7, 255–277. [Google Scholar] [CrossRef] [PubMed]
- Luzi, F.; Dominici, F.; Armentano, I.; Fortunati, E.; Burgos, N.; Fiori, S.; Jiménez, A.; Kenny, J.M.; Torre, L. Combined effect of cellulose nanocrystals, carvacrol and oligomeric lactic acid in PLA_PHB polymeric films. Carbohydr. Polym. 2019, 223, 115131. [Google Scholar] [CrossRef] [PubMed]
- Weng, Y.-X.; Wang, L.; Zhang, M.; Wang, X.-L.; Wang, Y.-Z. Biodegradation behavior of P(3HB,4HB)/PLA blends in real soil environments. Polym. Test. 2013, 32, 60–70. [Google Scholar] [CrossRef]
- Kale, G.; Auras, R.; Singh, S.P. Comparison of the degradability of poly(lactide) packages in composting and ambient exposure conditions. Packag. Technol. Sci. 2007, 20, 49–70. [Google Scholar] [CrossRef]
- Fabbri, M.; Soccio, M.; Costa, M.; Lotti, N.; Gazzano, M.; Siracusa, V.; Gamberini, R.; Rimini, B.; Munari, A.; García-Fernández, L.; et al. New fully bio-based PLLA triblock copoly(ester urethane)s as potential candidates for soft tissue engineering. Polym. Degrad. Stab. 2016, 132, 169–180. [Google Scholar] [CrossRef]
- Shah, A.A.; Hasan, F.; Hameed, A.; Ahmed, S. Biological degradation of plastics: A comprehensive review. Biotechnol. Adv. 2008, 26, 246–265. [Google Scholar] [CrossRef] [PubMed]
- Fortunati, E.; Rinaldi, S.; Peltzer, M.; Bloise, N.; Visai, L.; Armentano, I.; Jiménez, A.; Latterini, L.; Kenny, J.M. Nano-biocomposite films with modified cellulose nanocrystals and synthesized silver nanoparticles. Carbohydr. Polym. 2014, 101, 1122–1133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seoane, I.T.; Manfredi, L.B.; Cyras, V.P.; Torre, L.; Fortunati, E.; Puglia, D. Effect of cellulose nanocrystals and bacterial cellulose on disintegrability in composting conditions of plasticized PHB nanocomposites. Polymers 2017, 9, 561. [Google Scholar] [CrossRef] [PubMed] [Green Version]











| PLA [wt%] | PHB [wt%] | TB [wt%] | ChNP [wt%] | |
|---|---|---|---|---|
| PLA | 100 | / | / | / |
| PLA/TB | 85 | / | 15 | / |
| PHB | / | 100 | / | / |
| PHB/TB | / | 85 | 15 | / |
| PLA/PHB | 60 | 40 | / | / |
| PLA/PHB/TB | 51 | 35 | 15 | / |
| PLA/PHB/TB/ChNP | 49.8 | 33.2 | 15 | 2 |
| Average Length, L [nm] | Average Diameter, d [nm] | Aspect Ratio, L:d |
|---|---|---|
| 300 (170) | 40 (10) | 7.5:1 |
| Thickness [μm] | Op [%] | |||||
|---|---|---|---|---|---|---|
| White Control | 92.28 (0.00) | 1.38 (0.00) | −0.86 (0.00) | |||
| PLA | 118 (5) | 94.21 (0.08) | −1.15 (0.01) | 0.11 (0.03) | 2.09 (0.09) | 8.5 (0.1) |
| PLA/TB | 119 (4) | 93.37 (0.30) | −1.18 (0.02) | 0.49 (0.09) | 1.16 (0.30) | 8.4 (0.5) |
| PHB | 108 (2) | 85.14 (1.24) | 0.47 (0.25) | 17.01 (1.93) | 17.68 (2.25) | 16.5 (1.8) |
| PHB/TB | 111 (13) | 89.15 (0.55) | −0.85 (0.13) | 10.76 (1.79) | 10.40 (1.87) | 12.7 (0.2) |
| PLA/PHB | 122 (17) | 92.60 (0.52) | −1.44 (0.25) | 4.97 (0.82) | 4.15 (0.78) | 18.1 (1.5) |
| PLA/PHB/TB | 114 (9) | 90.00 (2.50) | −1.16 (0.25) | 7.38 (3.21) | 7.01 (3.81) | 13.3 (1.9) |
| PLA/PHB/TB/ChNP | 233 (32) | 89.00 (1.06) | −1.12 (0.14) | 11.82 (1.93) | 11.45 (2.15) | 12.9 (0.6) |
| DSC (I Scan) | TG | DTG | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tg,PLA [°C] | Tc,PLA [°C] | ΔHc,PLA [J g−1] | Tm,PLA [°C] | ΔHm,PLA [J g−1] | Tm,PHB [°C] | ΔHm,PHB [J g−1] | χc,PLA [%] | χc,PHB [%] | T0 [°C] | Tmax,PHB [°C] | Tmax,PLA [°C] | |
| PLA | 62 | 124 | 2.5 | 152 | 3.3 | 0.9 | 322 | 363 | ||||
| PLA/TB | 46 | 101 | 25.0 | 151 | 25.5 | 0.6 | 254 | 353 | ||||
| PHB | 173 | 85.3 | 58.4 | 250 | 277 | |||||||
| PHB/TB | 168 | 63.9 | 51.5 | 210 | 268 | |||||||
| PLA/PHB | 62 | 122 | 6.8 | 152 | 22.9 | 171 | 27.4 | 28.8 | 46.8 | 258 | 279 | 351 |
| PLA/PHB/TB | 29 | 77 | 12.0 | 146 | 21.8 | 165 | 23.4 | 20.7 | 47.2 | 208 | 278 | 358 |
| PLA/PHB/TB/ChNP | 27 | 74 | 9.1 | 146 | 20.1 | 165 | 26.0 | 23.8 | 53.7 | 214 | 277 | 337 |
| Mechanical Properties | Barrier Properties | Migration Studies | ||||||
|---|---|---|---|---|---|---|---|---|
| E [MPa] | σmax [MPa] | εb [%] | WVP ∗ 1011 [g/s m Pa] | GTR [cm3cm/m2 d atm] | Isooctane [mg dm−2] | Ethanol 10% (v/v) [mg dm−2] | ||
| O2 | CO2 | |||||||
| PLA | 2700 (160) | 55 (4) | 5 (2) | 2.2 (0.1) | 3.5 (0.1) | 4.0 (0.2) | 1.7 (0.6) | 8.1 (1.8) |
| PLA/TB | 2280 (140) | 31 (1) | 6 (2) | 3.0 (0.1) | 4.2 (0.2) | 4.3 (0.2) | 2.3 (0.9) | 14.9 (1.5) |
| PHB | 2230 (130) | 34 (2) | 4 (1) | 0.6 (0.05) | 1.1 (0.05) | 2.8 (0.1) | 2.7 (0.8) | 6.7 (0.5) |
| PHB/TB | 880 (50) | 15 (1) | 2 (1) | 2.8 (0.7) | 21.1 (0.8) | 26.0 (1.0) | 5.0 (1.0) | 14.5 (0.1) |
| PLA/PHB | 2630 (160) | 33 (2) | 2 (0) | 0.8 (0.1) | 1.0 (0.04) | 1.6 (0.06) | 3.2 (1.6) | 7.2 (2.0) |
| PLA/PHB/TB | 960 (60) | 12 (1) | 67 (6) | 2.2 (0.2) | 12.6 (0.5) | 19.3 (0.7) | 8.2 (1.7) | 13.7 (2.6) |
| PLA/PHB/TB/ChNP | 2440 (150) | 14 (1) | 4 (1) | 2.9 (0.1) | 6.7 (0.3) | 17.3 (0.6) | 6.7 (2.0) | 9.0 (1.8) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iglesias-Montes, M.L.; Soccio, M.; Siracusa, V.; Gazzano, M.; Lotti, N.; Cyras, V.P.; Manfredi, L.B. Chitin Nanocomposite Based on Plasticized Poly(lactic acid)/Poly(3-hydroxybutyrate) (PLA/PHB) Blends as Fully Biodegradable Packaging Materials. Polymers 2022, 14, 3177. https://doi.org/10.3390/polym14153177
Iglesias-Montes ML, Soccio M, Siracusa V, Gazzano M, Lotti N, Cyras VP, Manfredi LB. Chitin Nanocomposite Based on Plasticized Poly(lactic acid)/Poly(3-hydroxybutyrate) (PLA/PHB) Blends as Fully Biodegradable Packaging Materials. Polymers. 2022; 14(15):3177. https://doi.org/10.3390/polym14153177
Chicago/Turabian StyleIglesias-Montes, Magdalena L., Michelina Soccio, Valentina Siracusa, Massimo Gazzano, Nadia Lotti, Viviana P. Cyras, and Liliana B. Manfredi. 2022. "Chitin Nanocomposite Based on Plasticized Poly(lactic acid)/Poly(3-hydroxybutyrate) (PLA/PHB) Blends as Fully Biodegradable Packaging Materials" Polymers 14, no. 15: 3177. https://doi.org/10.3390/polym14153177