Acetylation Modification, Characterization, and Anticomplementary Activity of Polysaccharides from Rhododendron dauricum Leaves
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Polysaccharides and Their Acetylated Derivatives
2.3. Degree of Substitution Measurement
2.4. Single Factor and Response Surface Design of Acetylation Modification
2.5. Structural Characterization
2.5.1. Fourier Transform Infrared (FT-IR) Analysis
2.5.2. Monosaccharide Composition Analysis
2.5.3. Molecular Weight Analysis
2.5.4. Congo Red Test
2.6. Anticomplementary Activity of Polysacchairdes
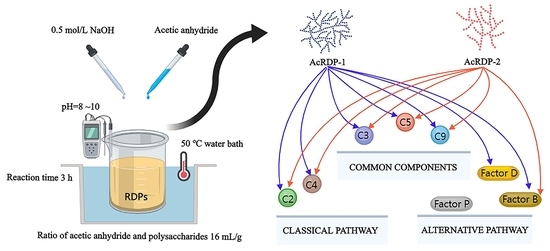
2.7. Determination of Complement Targets
2.8. Statistical Analysis
3. Results and Discussion
3.1. Single Factor Analysis
3.2. RSM Optimization of Acetylation Modification
3.2.1. Model Fitting
3.2.2. Response Surface Analysis
3.2.3. Verification of the Predictive Model
3.3. Structural Characterization of AcRDP-1 and AcRDP-2
3.3.1. FT-IR Analysis
3.3.2. Monosaccharide Composition Analysis
3.3.3. Molecular Weight Analysis
3.3.4. Congo Red Test
3.4. Anticomplementary Activity Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, J.M.; Jin, M.; Jin, C.S.; Ye, C.; Zhou, Y.; Wang, R.S.; Cui, H.H.; Zhou, W.; Li, G. A new pentacyclic triterpenoid from the leaves of Rhododendron dauricum L. with inhibition of NO production in LPS-induced RAW 264.7 cells. Nat. Prod. Res. 2020, 34, 3313–3319. [Google Scholar] [CrossRef] [PubMed]
- Ye, C.; Jin, M.; Jin, C.S.; Wang, R.S.; Wang, J.M.; Zhang, Y.; Li, S.N.; Sun, J.F.; Zhou, W.; Li, G. Two novel flavonoids from the leaves of Rhododendron dauricum L. with their inhibition of TNF-α production in LPS-induced RAW 264.7 cells. Nat. Prod. Res. 2021, 35, 1331–1339. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.Y.; Yu, R.Y.; Sun, J.F.; Duan, Y.Q.; Zhou, H.L.; Zhou, W.; Li, G. Static decolorization of polysaccharides from the leaves of Rhododendron dauricum: Process optimization, characterization and antioxidant activities. Process Biochem. 2022, 121, 113–125. [Google Scholar] [CrossRef]
- Xu, Y.; Wu, Y.J.; Sun, P.L.; Zhang, F.M.; Linhardt, R.J.; Zhang, A.Q. Chemically modified polysaccharides: Synthesis, characterization, structure activity relationships of action. Int. J. Biol. Macromol. 2019, 132, 970–977. [Google Scholar] [CrossRef] [PubMed]
- Li, S.J.; Xiong, Q.P.; Lai, X.P.; Li, X.; Wan, M.J.; Zhang, J.N.; Yan, Y.J.; Cao, M.; Lu, L.; Guan, J.M.; et al. Molecular modification of polysaccharides and resulting bioactivities. Compr. Rev. Food Sci. Food Saf. 2016, 15, 237–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, J.H.; Zhang, F.; Wang, Z.J.; Shen, M.Y.; Nie, S.P.; Xie, M.Y. Preparation, characterization and antioxidant activities of acetylated polysaccharides from Cyclocarya paliurus leaves. Carbohydr. Polym. 2015, 133, 596–604. [Google Scholar] [CrossRef] [PubMed]
- Hitri, K.; Kuttel, M.M.; Benedetto, G.D.; Lockyer, K.; Gao, F.; Hansal, P.; Rudd, T.R.; Beamish, E.; Rijpkema, S.; Ravenscroft, N.; et al. O-acetylation of typhoid capsular polysaccharide confers polysaccharide rigidity and immunodominance by masking additional epitopes. Vaccine 2019, 37, 3866–3875. [Google Scholar] [CrossRef]
- Guo, Q.B.; Liu, Y.; Cui, S.W. Structure, classification and modification of polysaccharides. Compr. Glycosci. 2021, 1, 204–219. [Google Scholar] [CrossRef]
- Ferreira, V.P.; Pangburn, M.K.; Cortés, C. Complement control protein factor H: The good, the bad, and the inadequate. Mol. Immunol. 2010, 47, 2187–2197. [Google Scholar] [CrossRef] [Green Version]
- Carroll, M.C. The complement system in regulation of adaptive immunity. Nat. Immunol. 2004, 5, 981–986. [Google Scholar] [CrossRef]
- Lutsep, H.L.; Clark, W.M. Current status of neuroprotective agents in the treatment of acute ischemic stroke. Curr. Neurol. Neurosci. Rep. 2001, 1, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Ricklin, V.D.; Hajishengallis, G.; Yang, K.; Lambris, J.D. Complement: A key system for immune surveillance and homeostasis. Nat. Immunol. 2010, 11, 785–797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harboe, M.; Thorgersen, E.B.; Mollnes, T.E. Advances in assay of complement function and activation. Adv. Drug Deliv. Rev. 2011, 63, 976–987. [Google Scholar] [CrossRef]
- Kim, D.M.; Kim, Y.; Seo, J.W.; Lee, J.; Park, U.; Ha, N.Y.; Koh, J.; Park, H.; Lee, J.W.; Ro, H.J.; et al. Enhanced eosinophil-mediated inflammation associated with antibody and complement-dependent pneumonic insults in critical COVID-19. Cell Rep. 2021, 37, 109798. [Google Scholar] [CrossRef]
- Cugno, M.; Meroni, P.L.; Gualtierotti, R.; Griffini, S.; Grovetti, E.; Torri, A.; Panigada, M.; Aliberti, S.; Blasi, F.; Tedesco, F.; et al. Complement activation in patients with COVID-19: A novel therapeutic target. J. Allergy Clin. Immunol. 2020, 146, 215–217. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Ji, D.; Zhu, M.X.; Chen, D.F.; Lu, Y. Juniperus pingii var. wilsonii acidic polysaccharide: Extraction, characterization and anticomplement activity. Carbohydr. Polym. 2020, 231, 115728. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Li, B.B.; Lu, Y.; Chen, D.F. Structural characterization and anticomplement activity of an acidic polysaccharide containing 3-O-methyl galactose from Juniperus tibetica. Int. J. Biol. Macromol. 2019, 132, 1244–1251. [Google Scholar] [CrossRef] [PubMed]
- Huo, J.Y.; Lu, Y.; Jiao, Y.K.; Chen, D.F. Structural characterization and anticomplement activity of an acidic polysaccharide from Hedyotis diffusa. Int. J. Biol. Macromol. 2020, 155, 1553–1560. [Google Scholar] [CrossRef]
- Huang, Z.; Zong, M.H.; Lou, W.Y. Effect of acetylation modification on the emulsifying and antioxidant properties of polysaccharide from Millettia speciosa Champ. Food Hydrocoll. 2022, 124, 107217. [Google Scholar] [CrossRef]
- Notari, R.E.; Munson, J.W. Hydroxamic acids I: Factors affecting the stability of the hydroxamic acid-iron complex. J. Pharm. Sci. 1969, 58, 1060–1064. [Google Scholar] [CrossRef]
- Song, Y.; Yang, Y.; Zhang, Y.Y.; Duan, L.S.; Zhou, C.L.; Ni, Y.Y.; Liao, X.J.; Li, Q.H.; Hu, X.S. Effect of acetylation on antioxidant and cytoprotective activity of polysaccharides isolated from pumpkin (Cucurbita pepo, lady godiva). Carbohyd. Polym. 2013, 98, 686–691. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.Y.; Zhou, H.L.; Zhao, J.L.; Sun, J.Q.; Li, M.; Sun, X.S. Microwave-assisted extraction, characterization and immunomodulatory activity on RAW264.7 cells of polysaccharides from Trichosanthes kirilowii Maxim seeds. Int. J. Biol. Macromol. 2020, 164, 2861–2872. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.Y.; Wang, P.H.; Zhou, H.L.; Li, Y.P. Extraction, characterization and in vitro antioxidant activity of polysaccharides from Carex meyeriana Kunth using different methods. Int. J. Biol. Macromol. 2018, 120, 2155–2164. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.Z.; Meng, M.; Duan, S.Q.; Feng, C.C.; Wang, C.L. Structure characterization, physicochemical property and immunomodulatory activity on RAW264.7 cells of a novel triple-helix polysaccharide from Craterellus cornucopioides. Int. J. Biol. Macromol. 2019, 126, 796–804. [Google Scholar] [CrossRef]
- Wang, R.S.; Sun, J.F.; Jin, M.; Ye, C.; Wang, J.M.; Jin, L.; Ma, Y.J.; Zhou, W.; Li, G. Two new phenolic glycosides with anti-complementary activity from the roots of Sanguisorba officinalis L. Nat. Prod. Res. 2021, 35, 4423–4432. [Google Scholar] [CrossRef]
- Ji, H.Y.; Dai, K.Y.; Liu, C.; Yu, J.; Liu, A.J.; Chen, Y.F. The ethanol-extracted polysaccharide from Cynanchum paniculatum: Optimization, structure, antioxidant and antitumor effects. Ind. Crop. Prod. 2022, 175, 114243. [Google Scholar] [CrossRef]
- Chen, X.H.; Wang, Z.R.; Kan, J.Q. Polysaccharides from ginger stems and leaves: Effects of dual and triple frequency ultrasound assisted extraction on structural characteristics and biological activities. Food Biosci. 2021, 42, 101166. [Google Scholar] [CrossRef]
- Wu, H.Y.; Li, M.M.; Yang, X.R.; Wei, Q.; Sun, L.Z.Y.; Zhao, J.C.; Shang, H.M. Extraction optimization, physicochemical properties and antioxidant and hypoglycemic activities of polysaccharides from roxburgh rose (Rosa roxburghii Tratt.) leaves. Int. J. Biol. Macromol. 2020, 165, 517–529. [Google Scholar] [CrossRef]
- Gong, T.; Liu, S.L.; Wang, H.Z.; Zhang, M. Supercritical CO2 fluid extraction, physicochemical properties, antioxidant activities and hypoglycemic activity of polysaccharides derived from fallen Ginkgo leaves. Food Biosci. 2021, 42, 101153. [Google Scholar] [CrossRef]
- Liu, W.; Lv, X.; Huang, W.H.; Yao, W.B.; Gao, X.D. Characterization and hypoglycemic effect of a neutral polysaccharide extracted from the residue of Codonopsis pilosula. Carbohydr. Polym. 2018, 197, 215–226. [Google Scholar] [CrossRef]
- Dong, X.; Zhu, C.P.; Huang, G.Q.; Xiao, J.X. Fractionation and structural characterization of polysaccharides derived from red grape pomace. Process Biochem. 2021, 109, 37–45. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, H.; Wang, Y.X.; Nie, S.P.; Li, C.; Xie, M.Y. Acetylation and carboxymethylation of the polysaccharide from Ganoderma atrum and their antioxidant and immunomodulating activities. Food Chem. 2014, 156, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Bu, W.; Dai, W.W.; Liu, H.M.; Bu, H.M.; Ju, X.Y.; Li, R.P.; Yuan, B. Structural characterization of a polysaccharide from Dioscorea opposita and assessment of its hepatoprotective activity. Process Biochem. 2022, 120, 156–168. [Google Scholar] [CrossRef]
- Li, J.J.; Hu, X.Z.; Li, X.P.; Ma, Z. Effects of acetylation on the emulsifying properties of Artemisia sphaerocephala Krasch. polysaccharide. Carbohydr. Polym. 2016, 144, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Schols, H.A.; Jin, Z.; Sulmann, E.; Voragen, A.G.J. Characterization of differently sized granule fractions of yellow pea, cowpea and chickpea starches after modification with acetic anhydride and vinyl acetate. Carbohydr. Polym. 2007, 67, 11–20. [Google Scholar] [CrossRef]
- Guo, X.Y.; Kang, J.; Xu, Z.Y.; Guo, Q.B.; Zhang, L.F.; Ning, H.F.; Cui, S.E. Triple-helix polysaccharides: Formation mechanisms and analytical methods. Carbohydr. Polym. 2021, 262, 117962. [Google Scholar] [CrossRef]
- Wang, X.M.; Zhang, Z.S.; Yao, Q.; Zhao, M.X.; Qi, H.M. Phosphorylation of low-molecular-weight polysaccharide from Enteromorpha linza with antioxidant activity. Carbohydr. Polym. 2013, 96, 371–375. [Google Scholar] [CrossRef]
- Eceker, E.E.; Lopez Castro, G. Complement and opsonic activities of fresh human sera. J. Immunol. 1947, 55, 169–181. [Google Scholar]
- Weiler, J.M.; Yurt, R.W.; Fearon, D.T.; Austen, K.F. Modulation of the formation of the amplification convertase of complement, C3b, Bb, by native and commercial heparin. J. Exp. Med. 1978, 147, 409–421. [Google Scholar] [CrossRef] [Green Version]
- Baker, P.J.; Lint, T.F.; McLeod, B.C.; Behrends, C.L.; Gewurz, H. Studies on the inhibition of C56-induced lysis (reactive lysis). VI. Modulation of C56-induced lysis polyanions and polycations. J. Immunol. 1975, 114, 554–558. [Google Scholar] [PubMed]
- Hughes-Jones, N.C.; Gardner, B. The reaction between the complement subcomponent C1q, IgG complexes and polyionic molecules. Immunology 1978, 34, 459–463. [Google Scholar] [PubMed]
- Almeda, S.; Rosenberg, R.D.; Bing, D.H. The binding properties of human complement component C1q. Interaction with mucopolysaccharides. J. Biol. Chem. 1983, 258, 785–791. [Google Scholar] [CrossRef]
- Weiler, J.M. Polyions regulate the alternative amplifification pathway of complement. Immunopharmacology 1983, 6, 245–255. [Google Scholar] [CrossRef]
- Weiler, J.M.; Edens, R.E.; Linhardt, R.J.; Kapelanski, D.P. Heparin and modifified heparin inhibit complement activation in vivo. J. Immunol. 1992, 148, 3210–3215. [Google Scholar]
- Fabris, F.; Luzzatto, G.; Stefanl, P.M. Heparin-induced thrombocytopenia. Haematologica 2000, 85, 72–81. [Google Scholar] [CrossRef]
- Fan, L.H.; Jiang, L.; Xu, Y.M.; Zhou, Y.; Shen, Y.; Xie, W.G.; Long, Z.H.; Zhou, J.P. Synthesis and anticoagulant activity of sodium alginate sulfates. Carbohydr. Polym. 2011, 83, 1797–1803. [Google Scholar] [CrossRef]







| Run | Reaction Time (X1, h) | Reaction Temperature (X2, °C) | Liquid-Solid Ratio (X3, mL/g) | Degree of Substitution | |
|---|---|---|---|---|---|
| Actual Value | Predicted Value | ||||
| 1 | 3 (0) | 60 (1) | 12 (−1) | 0.34 | 0.34 |
| 2 | 5 (1) | 50 (0) | 12 (−1) | 0.16 | 0.17 |
| 3 | 1 (−1) | 60 (1) | 16 (0) | 0.34 | 0.35 |
| 4 | 3 (0) | 50 (0) | 16 (0) | 0.46 | 0.47 |
| 5 | 3 (0) | 50 (0) | 16 (0) | 0.47 | 0.47 |
| 6 | 5 (1) | 40 (−1) | 16 (0) | 0.35 | 0.35 |
| 7 | 5 (1) | 50 (0) | 20 (1) | 0.36 | 0.36 |
| 8 | 5 (1) | 60 (1) | 16 (0) | 0.34 | 0.33 |
| 9 | 1 (−1) | 40 (−1) | 16 (0) | 0.30 | 0.31 |
| 10 | 3 (0) | 60 (1) | 20 (1) | 0.14 | 0.15 |
| 11 | 3 (0) | 50 (0) | 16 (0) | 0.46 | 0.47 |
| 12 | 3 (0) | 50 (0) | 16 (0) | 0.481 | 0.47 |
| 13 | 1 (−1) | 50 (0) | 12 (−1) | 0.35 | 0.36 |
| 14 | 3 (0) | 40 (−1) | 20 (1) | 0.33 | 0.33 |
| 15 | 3 (0) | 50 (0) | 16 (0) | 0.47 | 0.47 |
| 16 | 1 (−1) | 50 (0) | 20 (1) | 0.12 | 0.12 |
| 17 | 3 (0) | 40 (−1) | 12 (−1) | 0.19 | 0.18 |
| Source | Sum of Squares | Df | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 0.23 | 9 | 0.025 | 238.69 | <0.0001 ** |
| X1 | 0.001033 | 1 | 0.001033 | 9.74 | 0.0168 * |
| X2 | 0.0000000677 | 1 | 0000000677 | 0.0006385 | 0.9805 |
| X3 | 0.0009834 | 1 | 0.0009834 | 9.27 | 0.0243 * |
| X1X2 | 0.0007224 | 1 | 0.0007224 | 6.81 | 0.0349 * |
| X1X3 | 0.047 | 1 | 0.047 | 438.82 | <0.0001 ** |
| X2X3 | 0.030 | 1 | 0.030 | 283.14 | <0.0001 ** |
| X12 | 0.020 | 1 | 0.020 | 187.10 | <0.0001 ** |
| X22 | 0.019 | 1 | 0.019 | 181.79 | <0.0001 ** |
| X32 | 0.097 | 1 | 0.097 | 911.72 | <0.0001 ** |
| Residual | 0.0007422 | 7 | 0.022 | — | — |
| Lack of Fit | 0.0004664 | 3 | 0001555 | 2.25 | 0.2242 |
| Pure Error | 0.0002758 | 4 | 0.00006896 | — | — |
| Cor Total | 0.23 | 16 | — | — | — |
| R2 | 0.9968 | — | Adj R2 | 0.9926 | — |
| C.V. % | 3.06 | — | Pred R2 | 0.9655 | — |
| Samples | CH50 (mg/mL) | AP50 (mg/mL) |
|---|---|---|
| RDP-1 | NE | NE |
| RDP-2 | 0.870 ± 0.030 a | NE |
| AcRDP-1 | 0.009 ± 0.003 c | 0.015 ± 0.003 b |
| AcRDP-2 | 0.004 ± 0.001 c | 0.028 ± 0.005 b |
| Heparin | 0.114 ± 0.013 b | 0.138 ± 0.012 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Z.; Sun, J.; Jin, L.; Zong, T.; Duan, Y.; Zhou, H.; Zhou, W.; Li, G. Acetylation Modification, Characterization, and Anticomplementary Activity of Polysaccharides from Rhododendron dauricum Leaves. Polymers 2022, 14, 3130. https://doi.org/10.3390/polym14153130
Hu Z, Sun J, Jin L, Zong T, Duan Y, Zhou H, Zhou W, Li G. Acetylation Modification, Characterization, and Anticomplementary Activity of Polysaccharides from Rhododendron dauricum Leaves. Polymers. 2022; 14(15):3130. https://doi.org/10.3390/polym14153130
Chicago/Turabian StyleHu, Zhengyu, Jinfeng Sun, Long Jin, Tieqiang Zong, Yuanqi Duan, Hongli Zhou, Wei Zhou, and Gao Li. 2022. "Acetylation Modification, Characterization, and Anticomplementary Activity of Polysaccharides from Rhododendron dauricum Leaves" Polymers 14, no. 15: 3130. https://doi.org/10.3390/polym14153130