In Situ Fluorescent Illumination of Microplastics in Water Utilizing a Combination of Dye/Surfactant and Quenching Techniques
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of Different MPs
2.3. MP Staining Procedures
2.4. Characterization
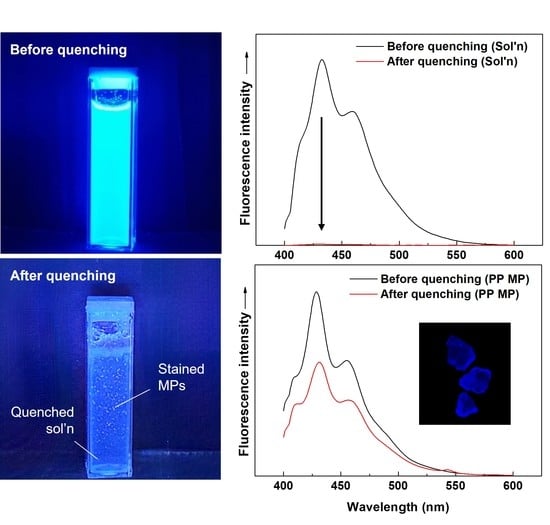
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Frias, J.; Nash, R. Microplastics: Finding a consensus on the definition. Mar. Pollut. Bull. 2019, 138, 145–147. [Google Scholar] [CrossRef] [PubMed]
- Andrady, A.L. The plastic in microplastics: A review. Mar. Pollut. Bull. 2017, 119, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Wright, S.L.; Kelly, F.J. Plastic and human health: A micro issue? Environ. Sci. Technol. 2017, 51, 6634–6647. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, H.; Paul Chen, J. Microplastics in freshwater systems: A review on occurrence, environmental effects, and methods for microplastics detection. Water Res. 2018, 137, 362–374. [Google Scholar] [CrossRef]
- Andrady, A.L. Microplastics in the marine environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef]
- Cella, C.; La Spina, R.; Mehn, D.; Fumagalli, F.; Ceccone, G.; Valsesia, A.; Gilliland, D. Detecting micro- and nanoplastics released from food packaging: Challenges and analytical strategies. Polymers 2022, 14, 1238. [Google Scholar] [CrossRef]
- GESAMP. Sources, Fate and Effects of Microplastics in the Marine Environment: A Global Assessment; International Maritime Organization: London, UK, 2015. [Google Scholar]
- Hartmann, N.B.; Huffer, T.; Thompson, R.C.; Hassellov, M.; Verschoor, A.; Daugaard, A.E.; Rist, S.; Karlsson, T.; Brennholt, N.; Cole, M.; et al. Are we speaking the same language? Recommendations for a definition and categorization framework for plastic debris. Environ. Sci. Technol. 2019, 53, 1039–1047. [Google Scholar] [CrossRef] [Green Version]
- Ziajahromi, S.; Kumar, A.; Neale, P.A.; Leusch, F.D.L. Environmentally relevant concentrations of polyethylene microplastics negatively impact the survival, growth and emergence of sediment-dwelling invertebrates. Environ. Pollut. 2018, 236, 425–431. [Google Scholar] [CrossRef]
- Raju, S.; Carbery, M.; Kuttykattil, A.; Senathirajah, K.; Subashchandrabose, S.R.; Evans, G.; Thavamani, P. Transport and fate of microplastics in wastewater treatment plants: Implications to environmental health. Rev. Environ. Sci. Biotechnol. 2018, 17, 637–653. [Google Scholar] [CrossRef]
- Cui, R.; Kim, S.W.; An, Y.J. Polystyrene nanoplastics inhibit reproduction and induce abnormal embryonic development in the freshwater crustacean Daphnia galeata. Sci. Rep. 2017, 7, 12095. [Google Scholar] [CrossRef]
- Yee, M.S.; Hii, L.W.; Looi, C.K.; Lim, W.M.; Wong, S.F.; Kok, Y.Y.; Tan, B.K.; Wong, C.Y.; Leong, C.O. Impact of microplastics and nanoplastics on human health. Nanomaterials 2021, 11, 496. [Google Scholar] [CrossRef]
- Huerta Lwanga, E.; Gertsen, H.; Gooren, H.; Peters, P.; Salanki, T.; van der Ploeg, M.; Besseling, E.; Koelmans, A.A.; Geissen, V. Microplastics in the terrestrial ecosystem: Implications for Lumbricus terrestris (Oligochaeta, Lumbricidae). Environ. Sci. Technol. 2016, 50, 2685–2691. [Google Scholar] [CrossRef]
- Azeem, I.; Adeel, M.; Ahmad, M.A.; Shakoor, N.; Jiangcuo, G.D.; Azeem, K.; Ishfaq, M.; Shakoor, A.; Ayaz, M.; Xu, M.; et al. Uptake and accumulation of nano/microplastics in plants: A critical review. Nanomaterials 2021, 11, 2935. [Google Scholar] [CrossRef]
- Monkul, M.M.; Ozhan, H.O. Microplastic contamination in soils: A review from geotechnical engineering view. Polymers 2021, 13, 4129. [Google Scholar] [CrossRef]
- Primpke, S.; Christiansen, S.H.; Cowger, W.; De Frond, H.; Deshpande, A.; Fischer, M.; Holland, E.B.; Meyns, M.; O’Donnell, B.A.; Ossmann, B.E.; et al. Critical assessment of analytical methods for the harmonized and cost-efficient analysis of microplastics. Appl. Spectrosc. 2020, 74, 1012–1047. [Google Scholar] [CrossRef]
- Zarfl, C. Promising techniques and open challenges for microplastic identification and quantification in environmental matrices. Anal. Bioanal. Chem. 2019, 411, 3743–3756. [Google Scholar] [CrossRef]
- Prata, J.C.; da Costa, J.P.; Duarte, A.C.; Rocha-Santos, T. Methods for sampling and detection of microplastics in water and sediment: A critical review. Trends Anal. Chem. 2019, 110, 150–159. [Google Scholar] [CrossRef]
- Silva, A.B.; Bastos, A.S.; Justino, C.I.L.; da Costa, J.P.; Duarte, A.C.; Rocha-Santos, T.A.P. Microplastics in the environment: Challenges in analytical chemistry—A review. Anal. Chim. Acta 2018, 1017, 1–19. [Google Scholar] [CrossRef]
- Shim, W.J.; Hong, S.H.; Eo, S.E. Identification methods in microplastic analysis: A review. Anal. Methods 2017, 9, 1384–1391. [Google Scholar] [CrossRef]
- Cerasa, M.; Teodori, S.; Pietrelli, L. Searching nanoplastics: From sampling to sample processing. Polymers 2021, 13, 3658. [Google Scholar] [CrossRef]
- Ziajahromi, S.; Neale, P.A.; Rintoul, L.; Leusch, F.D. Wastewater treatment plants as a pathway for microplastics: Development of a new approach to sample wastewater-based microplastics. Water Res. 2017, 112, 93–99. [Google Scholar] [CrossRef]
- Löder, M.G.J.; Gerdts, G. Methodology used for the detection and identification of microplastics—A critical appraisal. In Marine Anthropogenic Litter; Bergmann, M., Gutow, L., Klages, M., Eds.; Springer: Cham, Switzerland, 2015; pp. 201–227. [Google Scholar] [CrossRef] [Green Version]
- Lenz, R.; Enders, K.; Stedmon, C.A.; Mackenzie, D.M.A.; Nielsen, T.G. A critical assessment of visual identification of marine microplastic using Raman spectroscopy for analysis improvement. Mar. Pollut. Bull. 2015, 100, 82–91. [Google Scholar] [CrossRef]
- Shruti, V.C.; Perez-Guevara, F.; Roy, P.D.; Kutralam-Muniasamy, G. Analyzing microplastics with Nile Red: Emerging trends, challenges, and prospects. J. Hazard. Mater. 2022, 423, 127171. [Google Scholar] [CrossRef]
- Liu, S.; Shang, E.; Liu, J.; Wang, Y.; Bolan, N.; Kirkham, M.B.; Li, Y. What have we known so far for fluorescence staining and quantification of microplastics: A tutorial review. Front. Environ. Sci. Eng. 2022, 16, 8. [Google Scholar] [CrossRef]
- Capolungo, C.; Genovese, D.; Montalti, M.; Rampazzo, E.; Zaccheroni, N.; Prodi, L. Photoluminescence-based techniques for the detection of micro- and nanoplastics. Chem. Eur. J. 2021, 27, 17529–17541. [Google Scholar] [CrossRef]
- Stanton, T.; Johnson, M.; Nathanail, P.; Gomes, R.L.; Needham, T.; Burson, A. Exploring the efficacy of Nile red in microplastic quantification: A costaining approach. Environ. Sci. Technol. Lett. 2019, 6, 606–611. [Google Scholar] [CrossRef]
- Hengstmann, E.; Fischer, E.K. Nile red staining in microplastic analysis-proposal for a reliable and fast identification approach for large microplastics. Environ. Monit. Assess. 2019, 191, 612. [Google Scholar] [CrossRef]
- Maes, T.; Jessop, R.; Wellner, N.; Haupt, K.; Mayes, A.G. A rapid-screening approach to detect and quantify microplastics based on fluorescent tagging with Nile Red. Sci. Rep. 2017, 7, 44501. [Google Scholar] [CrossRef] [Green Version]
- Erni-Cassola, G.; Gibson, M.I.; Thompson, R.C.; Christie-Oleza, J.A. Lost, but found with Nile red: A novel method for detecting and quantifying small microplastics (1 mm to 20 μm) in environmental samples. Environ. Sci. Technol. 2017, 51, 13641–13648. [Google Scholar] [CrossRef] [Green Version]
- Shim, W.J.; Song, Y.K.; Hong, S.H.; Jang, M. Identification and quantification of microplastics using Nile Red staining. Mar. Pollut. Bull. 2016, 113, 469–476. [Google Scholar] [CrossRef]
- Karakolis, E.G.; Nguyen, B.; You, J.B.; Rochman, C.M.; Sinton, D. Fluorescent dyes for visualizing microplastic particles and fibers in laboratory-based studies. Environ. Sci. Technol. Lett. 2019, 6, 334–340. [Google Scholar] [CrossRef]
- Lv, L.; Qu, J.; Yu, Z.; Chen, D.; Zhou, C.; Hong, P.; Sun, S.; Li, C. A simple method for detecting and quantifying microplastics utilizing fluorescent dyes—Safranine T, fluorescein isophosphate, Nile red based on thermal expansion and contraction property. Environ. Pollut. 2019, 255, 113283. [Google Scholar] [CrossRef] [PubMed]
- Caponetti, V.; Mavridi-Printezi, A.; Cingolani, M.; Rampazzo, E.; Genovese, D.; Prodi, L.; Fabbri, D.; Montalti, M. A selective ratiometric fluorescent probe for no-wash detection of PVC microplastic. Polymers 2021, 13, 1588. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo-Ruz, V.; Gutow, L.; Thompson, R.C.; Thiel, M. Microplastics in the marine environment: A review of the methods used for identification and quantification. Environ. Sci. Technol. 2012, 46, 3060–3075. [Google Scholar] [CrossRef]
- Asamoah, B.O.; Salmi, P.; Raty, J.; Ryymin, K.; Talvitie, J.; Karjalainen, A.K.; Kukkonen, J.V.K.; Roussey, M.; Peiponen, K.E. Optical monitoring of microplastics filtrated from wastewater sludge and suspended in ethanol. Polymers 2021, 13, 871. [Google Scholar] [CrossRef]
- Colson, B.C.; Michel, A.P.M. Flow-through quantification of microplastics using impedance spectroscopy. ACS Sens. 2021, 6, 238–244. [Google Scholar] [CrossRef]
- Prata, J.C.; Reis, V.; Matos, J.T.V.; da Costa, J.P.; Duarte, A.C.; Rocha-Santos, T. A new approach for routine quantification of microplastics using Nile Red and automated software (MP-VAT). Sci. Total Environ. 2019, 690, 1277–1283. [Google Scholar] [CrossRef]
- Tehrani-Bagha, A.R.; Holmberg, K. Solubilization of hydrophobic dyes in surfactant solutions. Materials 2013, 6, 580–608. [Google Scholar] [CrossRef] [Green Version]
- Weiss, J.; Coupland, J.N.; McClements, D.J. Solubilization of hydrocarbon emulsion droplets suspended in nonionic surfactant micelle solutions. J. Phys. Chem. 1996, 100, 1066–1071. [Google Scholar] [CrossRef]
- Smith McWilliams, A.D.; Ergülen, S.; Ogle, M.M.; de los Reyes, C.A.; Pasquali, M.; Martí, A.A. Fluorescent surfactants from common dyes—Rhodamine B and Eosin Y. Pure Appl. Chem. 2019, 92, 265–274. [Google Scholar] [CrossRef]
- Weiss, J.; McClements, D.J. Mass transport phenomena in oil-in-water emulsions containing surfactant micelles: Solubilization. Langmuir 2000, 16, 5879–5883. [Google Scholar] [CrossRef]
- Winnik, F.M.; Regismond, S.T.J.C.; Physicochemical, S.A.; Aspects, E. Fluorescence methods in the study of the interactions of surfactants with polymers. Colloids Surf. A Physicochem. Eng. Asp. 1996, 118, 1–39. [Google Scholar] [CrossRef]
- Helenius, A.; McCaslin, D.R.; Fries, E.; Tanford, C. Properties of detergents. Methods Enzymol. 1979, 56, 734–749. [Google Scholar] [CrossRef]
- Koreiviene, J. Microalgae lipid staining with fluorescent BODIPY dye. Methods Mol. Biol. 2020, 1980, 47–53. [Google Scholar] [CrossRef]
- Yadigarli, A.; Song, Q.; Druzhinin, S.I.; Schonherr, H. Probing of local polarity in poly(methyl methacrylate) with the charge transfer transition in Nile red. Beilstein J. Org. Chem. 2019, 15, 2552–2562. [Google Scholar] [CrossRef] [Green Version]
- Gagne, F.; Auclair, J.; Quinn, B. Detection of polystyrene nanoplastics in biological samples based on the solvatochromic properties of Nile red: Application in Hydra attenuata exposed to nanoplastics. Environ. Sci. Pollut. Res. 2019, 26, 33524–33531. [Google Scholar] [CrossRef]
- Rumin, J.; Bonnefond, H.; Saint-Jean, B.; Rouxel, C.; Sciandra, A.; Bernard, O.; Cadoret, J.P.; Bougaran, G. The use of fluorescent Nile red and BODIPY for lipid measurement in microalgae. Biotechnol. Biofuels 2015, 8, 42. [Google Scholar] [CrossRef] [Green Version]
- Choi, T.-S.; Shimizu, Y.; Shirai, H.; Hamada, K. Disperse dyeing of nylon 6 fiber using gemini surfactants containing ammonium cations as auxiliaries. Dye. Pigment. 2001, 48, 217–226. [Google Scholar] [CrossRef]
- Zhegalova, N.G.; He, S.; Zhou, H.; Kim, D.M.; Berezin, M.Y. Minimization of self-quenching fluorescence on dyes conjugated to biomolecules with multiple labeling sites via asymmetrically charged NIR fluorophores. Contrast Media Mol. Imaging 2014, 9, 355–362. [Google Scholar] [CrossRef] [Green Version]
- Lakowicz, J.R.; Malicka, J.; D’Auria, S.; Gryczynski, I. Release of the self-quenching of fluorescence near silver metallic surfaces. Anal. Biochem. 2003, 320, 13–20. [Google Scholar] [CrossRef] [Green Version]
- Simon, M.; van Alst, N.; Vollertsen, J. Quantification of microplastic mass and removal rates at wastewater treatment plants applying Focal Plane Array (FPA)-based Fourier Transform Infrared (FT-IR) imaging. Water Res. 2018, 142, 1–9. [Google Scholar] [CrossRef]
- Anger, P.M.; von der Esch, E.; Baumann, T.; Elsner, M.; Niessner, R.; Ivleva, N.P. Raman microspectroscopy as a tool for microplastic particle analysis. Trends Anal. Chem. 2018, 109, 214–226. [Google Scholar] [CrossRef]
- Thipperudrappa, J.; Biradar, D.S.; Lagare, M.T.; Hanagodimath, S.M.; Inamdar, S.R.; Kadadevaramath, J.S. Fluorescence quenching of BPBD by aniline in benzene–acetonitrile mixtures. J. Photochem. Photobiol. A Chem. 2006, 177, 89–93. [Google Scholar] [CrossRef]
- Geethanjali, H.S.; Nagaraja, D.; Melavanki, R.M.; Kusanur, R.A. Fluorescence quenching of boronic acid derivatives by aniline in alcohols—A Negative deviation from Stern–Volmer equation. J. Lumin. 2015, 167, 216–221. [Google Scholar] [CrossRef]
- Tamminga, M.; Hengstmann, E.; Fischer, E.K. Nile Red staining as a subsidiary method for microplastic quantification: A comparison of three solvents and factors influencing application reliability. SDRP J. Earth Sci. Environ. Studies 2017, 2, 165–172. [Google Scholar] [CrossRef] [Green Version]
- Tong, H.; Jiang, Q.; Zhong, X.; Hu, X. Rhodamine B dye staining for visualizing microplastics in laboratory-based studies. Environ. Sci. Pollut. Res. 2021, 28, 4209–4215. [Google Scholar] [CrossRef]
- Wiggin, K.J.; Holland, E.B. Validation and application of cost and time effective methods for the detection of 3–500 μm sized microplastics in the urban marine and estuarine environments surrounding Long Beach, California. Mar. Pollut. Bull. 2019, 143, 152–162. [Google Scholar] [CrossRef]
- Tosic, T.N.; Vruggink, M.; Vesman, A. Microplastics quantification in surface waters of the Barents, Kara and White Seas. Mar. Pollut. Bull. 2020, 161, 111745. [Google Scholar] [CrossRef]
- Cook, S.; Chan, H.L.; Abolfathi, S.; Bending, G.D.; Schafer, H.; Pearson, J.M. Longitudinal dispersion of microplastics in aquatic flows using fluorometric techniques. Water Res. 2020, 170, 115337. [Google Scholar] [CrossRef]
- James, E.M. Polymer Data Handbook; Oxford University Press: New York, NY, USA, 1999. [Google Scholar]
- Merrington, A. 11—Recycling of Plastics. In Applied Plastics Engineering Handbook; Kutz, M., Ed.; William Andrew Publishing: Oxford, UK, 2011; pp. 177–192. [Google Scholar] [CrossRef]
- Adachi, K.; Takahashi, T.; Kamehashi, K.; Watanabe, K.; Uchiyama, K.; Kuriyama, T.; Miyata, K.; Hisamastu, T. Thermal Effects on Ultrasonic Joining of Thin Plastic Films Using Torsional Vibrations. Jpn. J. Appl. Phys. 2008, 47, 6431–6436. [Google Scholar] [CrossRef]
- Gent, M.; Sierra, H.M.; Menendez, M.; de Cos Juez, F.J. Evaluation of ground calcite/water heavy media cyclone suspensions for production of residual plastic concentrates. Waste Manag. 2018, 71, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.-W.; Youngstrom, C.R.; Agarwal, S.; Al-Mulla, A.; Gaggar, S.K.; Gupta, R.K. Novel Biomass-Based Non-Halogenated FR Styrenic Blends. In Fire and Polymers VI: New Advances in Flame Retardant Chemistry and Science; ACS Publications: Washington, DC, USA, 2012; pp. 151–165. [Google Scholar] [CrossRef]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, D.H.; Oh, S.B.; Hong, S.C. In Situ Fluorescent Illumination of Microplastics in Water Utilizing a Combination of Dye/Surfactant and Quenching Techniques. Polymers 2022, 14, 3084. https://doi.org/10.3390/polym14153084
Park DH, Oh SB, Hong SC. In Situ Fluorescent Illumination of Microplastics in Water Utilizing a Combination of Dye/Surfactant and Quenching Techniques. Polymers. 2022; 14(15):3084. https://doi.org/10.3390/polym14153084
Chicago/Turabian StylePark, Doo Hong, Se Bin Oh, and Sung Chul Hong. 2022. "In Situ Fluorescent Illumination of Microplastics in Water Utilizing a Combination of Dye/Surfactant and Quenching Techniques" Polymers 14, no. 15: 3084. https://doi.org/10.3390/polym14153084