Effect of Water Uptake, Adhesion and Anti-Corrosion Performance for Silicone-Epoxy Coatings Treated with GLYMO on 2024 Al-Alloy
Abstract
:1. Introduction
2. Experimental Details
2.1. Materials
2.2. Sample Preparation
2.3. Gravimetric Experiments
2.4. Electrochemical Measurements
2.5. Differential Scanning Calorimeter (DSC)
2.6. Pull-Off Adhesion and Salt Spray Tests
3. Results
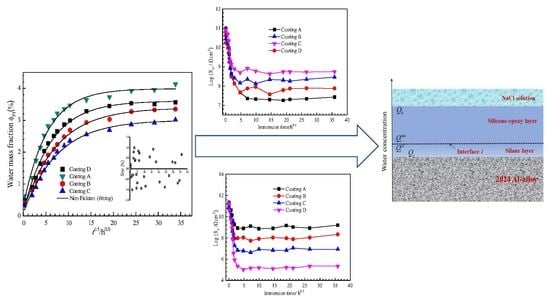
3.1. Water Uptake of the Coatings
3.2. Tg Variation after Water Permeation
3.3. Anti-Corrosion Performance of Silicone-Epoxy Coatings with GLYMO
3.4. Wet Adhesion and Salt Spray
3.5. SCEM
4. Discussion
5. Conclusions
- (1)
- Water uptake of silicone-epoxy coatings decreased after incorporated with GLYMO monomer. With the increase of GLYMO content, water of the coatings went through a minimum, corresponding to 1.5% of GLYMO monomer. EIS experiments also show that mixing a certain amount of GLYMO can improve the protective performance of silicone-epoxy coating.
- (2)
- For GLYMO-incorporated coatings, Tg increased slightly after water permeation because of the self-repairing effect, and finally decreased lower than that of the coating without GLYMO.
- (3)
- SECM measurement showed that the corrosion rate of Al-alloy substrate beneath the artificial defects of GLYMO-incorporated silicone-epoxy coatings was also significantly reduced.
- (4)
- Salt spray and adhesion tests showed the enhancement of interface structure. Adding GLYMO could preferentially form a micro-nano silane layer between the silicone-epoxy coating and Al-alloy. This was validated by the model of the water concentration jump.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Akbarinezhad, E.; Bahremandi, M.; Faridi, H.R.; Rezaei, F. Another approach for ranking and evaluating organic paint coatings via electrochemical impedance spectroscopy. Corros. Sci. 2009, 51, 356–363. [Google Scholar] [CrossRef]
- Allahar, K.N.; Hinderliter, B.R.; Tallman, D.E.; Bierwagen, G.P. Water transport in multilayer organic coatings. J. Electrochem. Soc. 2008, 155, F201. [Google Scholar] [CrossRef]
- Barranco, V.; Feliu, S., Jr.; Feliu, S. EIS study of the corrosion behaviour of zinc-based coatings on steel in quiescent 3% NaCl solution. Part 2: Coatings covered with an inhibitor-containing lacquer. Corros. Sci. 2004, 46, 2221–2240. [Google Scholar] [CrossRef] [Green Version]
- Brusciotti, F.; Snihirova, D.V.; Xue, H.; Montemor, M.F.; Lamaka, S.V.; Ferreira, M.G.S. Hybrid epoxy–silane coatings for improved corrosion protection of Mg alloy. Corros. Sci. 2013, 67, 82–90. [Google Scholar] [CrossRef]
- Castela, A.S.; Simões, A.M. Water sorption in freestanding PVC films by capacitance measurements. Prog. Org. Coat. 2003, 46, 130–134. [Google Scholar] [CrossRef]
- Foyet, A.; Wu, T.H.; van der Ven, L.; Kodentsov, A.; de With, G.; van Benthem, R. Influence of mixing ratio on the permeability of water and the corrosion performance of epoxy/amine coated un-pretreated Al-2024 evaluated by impedance spectroscopy. Prog. Org. Coat. 2009, 64, 138–141. [Google Scholar] [CrossRef]
- Hu, J.M.; Zhang, J.Q.; Cao, C.N. Determination of water uptake and diffusion of Cl− ion in epoxy primer on aluminium alloys in NaCl solution by electrochemical impedance spectroscopy. Prog. Org. Coat. 2003, 46, 273–279. [Google Scholar] [CrossRef]
- Jegdić, B.V.; Bajat, J.B.; Popić, J.P.; Stevanović, S.I.; Mišković-Stanković, V.B. The EIS investigation of powder polyester coatings on phosphated low carbon steel: The effect of NaNO2 in the phosphating bath. Corros. Sci. 2011, 53, 2872–2880. [Google Scholar] [CrossRef]
- Ji, W.-G.; Hu, J.-M.; Liu, L.; Zhang, J.-Q.; Cao, C.-N. Water uptake of epoxy coatings modified with γ-APS silane monomer. Prog. Org. Coat. 2006, 57, 439–443. [Google Scholar] [CrossRef]
- Ji, W.-G.; Hu, J.-M.; Zhang, J.-Q.; Cao, C.-N. Reducing the water absorption in epoxy coatings by silane monomer incorporation. Corros. Sci. 2006, 48, 3731–3739. [Google Scholar] [CrossRef]
- Lu, X.; Zuo, Y.; Zhao, X.; Tang, Y. The improved performance of a Mg-rich epoxy coating on AZ91D magnesium alloy by silane pretreatment. Corros. Sci. 2012, 60, 165–172. [Google Scholar] [CrossRef]
- Nguyen, V.N.; Perrin, F.X.; Vernet, J.L. Water permeability of organic/inorganic hybrid coatings prepared by sol-gel method: A comparison between gravimetric and capacitance measurements and evaluation of non-Fickian sorption models. Corros. Sci. 2005, 47, 397–412. [Google Scholar] [CrossRef]
- Qian, M.; Soutar, A.M.; Tan, X.H.; Zeng, X.T.; Wijesinghe, S.L. Two-part epoxy-siloxane hybrid corrosion protection coatings for carbon steel. Thin Solid Film. 2009, 517, 5237–5242. [Google Scholar] [CrossRef]
- Romano, A.-P.; Fedel, M.; Deflorian, F.; Olivie, M.-G. Silane sol-gel film as pretreatment for improvement of barrier properties and filiform corrosion resistance of 6016 aluminium alloy covered by cataphoretic coating. Prog. Org. Coat. 2011, 72, 695–702. [Google Scholar] [CrossRef]
- Tan, A.L.K.; Soutar, A.M. Hybrid sol-gel coatings for corrosion protection of copper. Thin Solid Film. 2008, 516, 5706–5709. [Google Scholar] [CrossRef]
- Tan, A.L.K.; Soutar, A.M.; Annergren, I.F.; Liu, Y.N. Multilayer sol-gel coatings for corrosion protection of magnesium. Surf. Coat. Technol. 2005, 198, 478–482. [Google Scholar] [CrossRef]
- Wang, D.; Bierwagen, G.P. Sol-gel coatings on metals for corrosion protection. Prog. Org. Coat. 2009, 64, 327–338. [Google Scholar] [CrossRef]
- Dang, D.N.; Peraudeau, B.; Cohendoz, S.; Mallarino, S.; Feaugas, X.; Touzain, S. Effect of mechanical stresses on epoxy coating ageing approached by Electrochemical Impedance Spectroscopy measurements. Electrochim. Acta. 2014, 124, 80–89. [Google Scholar] [CrossRef]
- Pathak, S.S.; Sharma, A.; Khanna, A.S. Value addition to waterborne polyurethane resin by silicone modification for developing high performance coating on aluminium alloy. Prog. Org. Coat. 2009, 65, 206–216. [Google Scholar] [CrossRef]
- Reuvers, N.J.W.; Huinink, H.P.; Adan, O.C.G.; Garcia, S.J.; Mol, J.M.C. Water uptake in thin nylon 6 films as measured by electrochemical impedance spectroscopy and magnetic resonance imaging. Electrochim. Acta. 2013, 94, 219–228. [Google Scholar] [CrossRef]
- Ji, W.-G.; Hu, J.-M.; Liu, L.; Zhang, J.-Q.; Cao, C.-N. Enhancement of corrosion performance of epoxy coatings by chemical modification with GPTMS silane monomer. J. Adhes. Sci. Technol. 2008, 22, 77–92. [Google Scholar] [CrossRef]
- Jiang, M.Y.; Wu, L.-K.; Hu, J.-M.; Zhang, J.-Q. Silane-incorporated epoxy coatings on aluminum alloy (AA2024). Part 1: Improved corrosion performance. Corros. Sci. 2015, 92, 118–126. [Google Scholar] [CrossRef]
- Zhang, J.T.; Hu, J.M.; Zhang, J.Q.; Cao, C.N. Studies of impedance models and water transport behaviours of polypropylene coated metals in NaCl solution. Prog. Org. Coat. 2004, 49, 293–301. [Google Scholar] [CrossRef]
- Zhang, J.-T.; Hu, J.-M.; Zhang, J.-Q.; Cao, C.-N. Studies of water transport behaviour and impedance models of epoxy-coated metals in NaCl solution by EIS. Prog. Org. Coat. 2004, 51, 145–151. [Google Scholar] [CrossRef]
- Zhou, Q.; Wang, Y.; Bierwagen, G.P. Influence of the composition of working fluids on flow-accelerated organic coating degradation: Deionized water versus electrolyte solution. Corros. Sci. 2012, 55, 97–106. [Google Scholar] [CrossRef]
- Zhu, C.; Xie, R.; Xue, J.; Song, L. Studies of the impedance models and water transport behaviours of cathodically polarised coating. Electrochim. Acta. 2011, 56, 5828–5835. [Google Scholar] [CrossRef]
- Zuo, Y.; Pang, R.; Li, W.; Xiong, J.P.; Tang, Y.M. The evaluation of coating performance by the variations of phase angles in middle and high-frequency domains of EIS. Corros. Sci. 2008, 50, 3322–3328. [Google Scholar] [CrossRef]
- Zeng, D.; Liu, Z.; Bai, S.; Zhao, J.; Wang, J. Corrosion resistance of bis-silane-modified epoxy coatings on an al-zn-mg-cu alloy. J. Mater. Eng. Perform. 2020, 29, 5282–5290. [Google Scholar] [CrossRef]
- Zeng, D.; Liu, Z.; Zou, L.; Wu, H. Corrosion resistance of epoxy coatings modified by bis-silane prepolymer on aluminium alloy. Coatings 2021, 11, 842. [Google Scholar] [CrossRef]
- Wang, P.; Schaefer, D.W. Why does Silane Enhance the Protective Properties of Epoxy Films? Langmuir 2008, 24, 13496–13501. [Google Scholar] [CrossRef]
- Souto, R.M.; Gonalez-Garcia, Y.; Gonzalez, S. In situ monitoring of electroactive species by using the scanning electrochemical microscope. Application to the investigation of degradation processes at defective coated metals. Corros. Sci. 2005, 47, 3312–3323. [Google Scholar] [CrossRef]
- Gonzalez, S.; Santana, J.J.; Gonzalez-Garcia, Y.; Fernandez-Merida, L.; Souto, R.M. Scanning electrochemical microscopy for the investigation of localized degradation processes in coated metals: Effect of oxygen. Corros. Sci. 2011, 53, 1910–1915. [Google Scholar] [CrossRef]
- Souto, R.M.; Gonzalez-Garcia, Y.; Gonzalez, S. Evaluation of the corrosion performance of coil-coated steel sheet as studied by scanning electrochemical microscopy. Corros. Sci. 2008, 50, 1637–1643. [Google Scholar] [CrossRef]
- Zadeh, M.A.; van der Zwaag, S.; Garcia, S.J. Adhesion and Long-Term Barrier Restoration of Intrinsic Self-Healing Hybrid Sol-Gel Coatings. ACS Appl. Mater. Interfaces. 2016, 8, 4126–4136. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Wu, L.; Hu, J.; Zhang, J. Silane-incorporated epoxy coatings on aluminum alloy (AA2024). Part 2: Mechanistic investigations. Corros. Sci. 2015, 92, 127–135. [Google Scholar] [CrossRef]
- Yuan, X.; Yue, Z.F.; Chen, X.; Wen, S.F.; Li, L.; Feng, T. The protective and adhesion properties of silicone-epoxy hybrid coatings on 2024 Al-alloy with a silane film as pretreatment. Corros. Sci. 2016, 104, 84–97. [Google Scholar] [CrossRef]













| Cu | Mg | Mn | Fe | Si | Zn | Ni | Ti | Al |
|---|---|---|---|---|---|---|---|---|
| 3.8 | 1.5 | 0.6 | 0.5 | 0.5 | 0.3 | 0.1 | 0.15 | balance |
| Coatings | D/ × 10−10 cm2·s−1 | Sc/ × 109 kg·cm·s−0.5 | 100 Vs/% |
|---|---|---|---|
| Coating A | 0.95 | 1.43 | 3.47 |
| Coating B | 0.55 | 1.17 | 2.69 |
| Coating C | 0.35 | 1.04 | 2.35 |
| Coating D | 0.47 | 1.26 | 2.99 |
| Sample | Tg/°C | ∆Tg/°C | ||
|---|---|---|---|---|
| Before Immersion | Immersion for 150 h | Immersion for 1050 h | ||
| Coating A | 92.8 | 87.4 | 65.3 | −27.5 |
| Coating B | 93.2 | 95.1 | 70.9 | −22.3 |
| Coating C | 95.8 | 96.4 | 75.3 | −20.5 |
| Coating D | 93.6 | 94.3 | 69.8 | −23.8 |
| Sample | Average Adhesion Strength (MPa) | Fracture Mode | |||
|---|---|---|---|---|---|
| Before Immersion | Coefficient of Variation, CV | After Immersion | Coefficient of Variation, CV | ||
| Coating A | 2.55 | 0.082 | 1.78 | 0.103 | 100% C/M |
| Coating B | 3.15 | 0.103 | 2.68 | 0.087 | |
| Coating C | 3.45 | 0.107 | 3.12 | 0.096 | |
| Coating D | 3.32 | 0.095 | 2.85 | 0.099 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, X.; Du, Y.; Lin, Z.; Liu, Z.; Gu, L. Effect of Water Uptake, Adhesion and Anti-Corrosion Performance for Silicone-Epoxy Coatings Treated with GLYMO on 2024 Al-Alloy. Polymers 2022, 14, 3076. https://doi.org/10.3390/polym14153076
Yuan X, Du Y, Lin Z, Liu Z, Gu L. Effect of Water Uptake, Adhesion and Anti-Corrosion Performance for Silicone-Epoxy Coatings Treated with GLYMO on 2024 Al-Alloy. Polymers. 2022; 14(15):3076. https://doi.org/10.3390/polym14153076
Chicago/Turabian StyleYuan, Xin, Yilin Du, Zhihai Lin, Zhiqiang Liu, and Lin Gu. 2022. "Effect of Water Uptake, Adhesion and Anti-Corrosion Performance for Silicone-Epoxy Coatings Treated with GLYMO on 2024 Al-Alloy" Polymers 14, no. 15: 3076. https://doi.org/10.3390/polym14153076