Hemicellulose Films from Curaua Fibers (Ananas erectifolius): Extraction and Thermal and Mechanical Characterization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
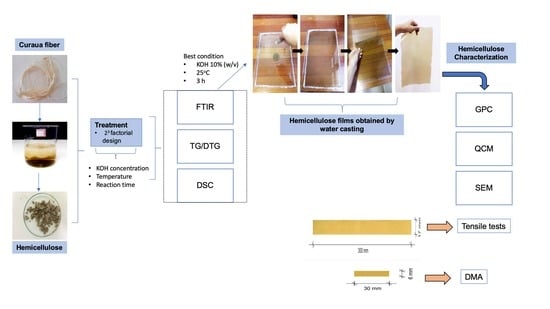
2.2. Hemicellulose Extraction
2.3. Hemicellulose Water Casting
2.4. Thermogravimetry (TG) and Differential Scanning Calorimetry (DSC)
2.5. Coupled Thermogravimetry–Fourier Transform Infrared Spectroscopy (TG-FTIR) Analysis
2.6. Scanning Electron Microscopy (SEM)
2.7. Molecular Weight of Hemicellulose Films by Gel Permeation Chromatography (GPC)
2.8. Quartz Crystal Microbalance (QCM)
2.9. Tensile Tests
2.10. Dynamic Mechanical Analysis (DMA)
3. Results and Discussion
3.1. Best Conditions from 23 Factorial Design
3.2. Thermal Characterization of Hemicellulose from 23 Factorial Design Characterization: TG-DTG/DSC
3.3. Coupled Thermogravimetry–Fourier Transform Infrared Spectroscopy (TG-FTIR)
3.4. Hemicellulose Film Characterization
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haider, T.P.; Völker, C.; Kramm, J.; Landfester, K.; Wurm, F.R. Plastics of the Future? The Impact of Biodegradable Polymers on the Environment and on Society. Angew. Chemie-Int. Ed. 2019, 58, 50–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arrieta, M.P.; Castro-López, M.D.M.; Rayón, E.; Barral-Losada, L.F.; López-Vilariño, J.M.; López, J.; González-Rodríguez, M.V. Plasticized poly (lactic acid)-poly (hydroxybutyrate) (PLA-PHB) blends incorporated with catechin intended for active food-packaging applications. J. Agric. Food Chem. 2014, 62, 10170–10180. [Google Scholar] [CrossRef] [PubMed]
- Arrieta, M.P.; Samper, M.D.; Aldas, M.; López, J. On the use of PLA-PHB blends for sustainable food packaging applications. Materials 2017, 10, 1008. [Google Scholar] [CrossRef]
- Baran, A.; Vrábel, P.; Olčák, D.; Chodák, I. Solid state 13C-NMR study of a plasticized PLA/PHB polymer blend. J. Appl. Polym. Sci. 2018, 135, 1–7. [Google Scholar] [CrossRef]
- Mendes, F.R.S.; Bastos, M.S.R.; Mendes, L.G.; Silva, A.R.A.; Sousa, F.D.; Monteiro-Moreira, A.C.O.; Cheng, H.N.; Biswas, A.; Moreira, R.A. Preparation and evaluation of hemicellulose films and their blends. Food Hydrocoll. 2017, 70, 181–190. [Google Scholar] [CrossRef] [Green Version]
- Muchlisyam; Silalahi, J.; Harahap, U. Hemicellulose: Isolation and Its Application in Pharmacy. In Handbook of Sustainable Polymers; Jenny Stanford Publishing: Unit Square, Singapore, 2016; pp. 338–373. [Google Scholar]
- Fengel, D.; Wegener, G. Wood: Chemistry, Ultrastructure, Reactions; Walter de Gruyter: New York, NY, USA, 2011; p. 618. [Google Scholar]
- AL-Oqla, F.M.; Salit, M.S. Materials Selection for Natural Fiber Composites; Woodhead Publishing: New Delhi, India, 2017; pp. 23–48. [Google Scholar]
- Rodrígues, B.S.; García, R.M.; Leão, R.M.; Amico, S.C.; Luz, S.M. Hemicellulose Removal in Curaua (Ananas erectifolius) Fibers for Polyester Composites. Nov. Sci. 2018, 10, 154–172. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, É.S.; Bueno, D.; Pagnocca, F.C.; Brienzo, M. Minor Biomass Particle Size for an Efficient Cellulose Accessibility and Enzymatic Hydrolysis. ChemistrySelect 2020, 5, 7627–7631. [Google Scholar] [CrossRef]
- Farhat, W.; Venditti, R.A.; Hubbe, M.; Taha, M.; Becquart, F.; Ayoub, A.A. Review of Water-Resistant Hemicellulose-Based Materials: Processing and Applications. ChemSusChem 2017, 10, 305–323. [Google Scholar] [CrossRef]
- Sjöström, E. Introduction to Carbohydrate Chemistry. In Wood Chemistry; Academic Press: Cambridge, MA, USA, 1993; pp. 21–50. [Google Scholar]
- Farhat, W.; Venditti, R.; Ayoub, A.; Prochazka, F.; Fernández-de-Alba, C.; Mignard, N.; Taha, M.; Becquart, F. Towards thermoplastic hemicellulose: Chemistry and characteristics of poly-(ε-caprolactone) grafting onto hemicellulose backbones. Mater. Des. 2018, 153, 298–307. [Google Scholar] [CrossRef]
- Hansen, N.M.L.; Plackett, D. Sustainable Films and Coatings from Hemicelluloses: A Review. Biomacromolecules 2008, 9, 1493–1505. [Google Scholar] [CrossRef]
- Mikkonen, K.S.; Tenkanen, M. Sustainable food-packaging materials based on future biorefinery products: Xylans and mannans. Trends Food Sci. Technol. 2012, 28, 90–102. [Google Scholar] [CrossRef]
- Oliveira, M.R.d. Hemicelulose de Fibras de Curauá (Ananas Erectifolius): Obtenção e Caracterização de Películas Poliméricas. Master’s Thesis, Universidade de Brasília, Brasília, Brazil, 2017; p. 53. [Google Scholar]
- Brienzo, M.; Siqueira, A.F.; Milagres, A.M.F. Search for optimum conditions of sugarcane bagasse hemicellulose extraction. Biochem. Eng. J. 2009, 46, 199–204. [Google Scholar] [CrossRef]
- Pardo, L.M.F.; Córdoba, A.G.; Galán, J.E.L. Evaluation of different methods for efficient extraction of hemicelluloses leaves and tops of sugarcane. DYNA 2018, 85, 18–27. [Google Scholar] [CrossRef]
- Bahcegul, E.; Ezgi, H.; Ozkan, N.; Bakir, U. Bioresource Technology Evaluation of alkaline pretreatment temperature on a multi-product basis for the co-production of glucose and hemicellulose based films from lignocellulosic biomass. Bioresour. Technol. 2012, 103, 440–445. [Google Scholar] [CrossRef] [PubMed]
- Rushing, H.; Karl, A.; Wisnowski, J. Design and Analysis of Experiments by Douglas Montgomery: A Supplement for Using JMP, 1st ed.; SAS Institute Inc.: Cary, NC, USA, 2013; ISBN 0-471-52000-4. [Google Scholar]
- Huang, X.; Elias, A.L. Sensors and Actuators B: Chemical Surface modified polymer thin films with enhanced sensitivity to a naphthenic acid model compound: A study by quartz crystal microbalance. Sens. Actuators B. Chem. 2014, 198, 7–14. [Google Scholar] [CrossRef]
- ASTM D882; Standard Test Method for Tensile Properties of Thin Plastic Sheeting. ASTM International: West Conshohocken, PA, USA, 2012; p. 12.
- Lopera-Valle, A.; Caputo, J.V.; Leão, R.; Sauvageau, D.; Luz, S.M.; Elias, A. Influence of epoxidized Canola Oil (eCO) and Cellulose Nanocrystals (CNCs) on the mechanical and thermal properties of polyhydroxybutyrate (PHB)-Poly (lactic acid) (PLA) blends. Polymers 2019, 11, 933. [Google Scholar] [CrossRef] [Green Version]
- da Silva Pieta, F.; Bariccatti, R. Response surface for sugars extracted from lignocellulosic material providing low energy expenditure. Semin. Ciências Exatas e Tecnológicas 2019, 40, 129. [Google Scholar] [CrossRef]
- Sunardi; Istikowati, W.T.; Sari, D.I. Extraction of α-cellulose from Eleocharis dulcis Holocellulose using NaOH and KOH. J. Phys. Conf. Ser. 2019, 1397, 012031. [Google Scholar] [CrossRef]
- Marques, V.; Melo, R.P.; Araujo, R.S.; Lunz, J.N.; Aguiar, D.O. Improvement of mechanical properties of natural fiber—Polypropylene composites using successive alkaline treatments. J. Appl. Polym. Sci. 2015, 132, 1–12. [Google Scholar] [CrossRef]
- Albinante, S.R.; Beatriz, É.; Vasques, A.; Lea, L.; Visconte, Y. A review on chemical treatment of natural fiber for mixing with polyolefins. Quím. Nova 2013, 36, 114–122. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; Yang, G.; Chen, J.; He, M. The Characterization of Hemicellulose Extract from Corn Stalk with Stepwise Alkali Extraction. J. Korea Tappi 2017, 49, 29–40. [Google Scholar] [CrossRef]
- Li, X.; Yu, D.; Zhang, W.; Li, Z.; Zhang, X.; Huang, H. Effective synthesis of cis-3-hexen-1-yl acetate via transesterification over KOH/γ-Al2O3: Structure and catalytic performance. Appl. Catal. A Gen. 2013, 455, 1–7. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Ren, J.; Sun, R.; Peng, F. Carboxymethylation of hemicelluloses isolated from sugarcane bagasse. Polym. Degrad. Stab. 2008, 93, 786–793. [Google Scholar] [CrossRef]
- Farhat, W.; Venditti, R.; Quick, A.; Taha, M.; Mignard, N.; Becquart, F.; Ayoub, A. Industrial Crops & Products Hemicellulose extraction and characterization for applications in paper coatings and adhesives. Ind. Crop. Prod. 2017, 107, 370–377. [Google Scholar]
- Figueroa, M.J.M.; De Moraes, P.D. Wood behavior at high temperatures. Ambient. Constr. 2021, 9, 157–174. [Google Scholar] [CrossRef]
- Alén, R.; Kotilainen, R.; Zaman, A. Thermochemical behavior of Norway spruce (Picea abies) at 180–225 °C. Wood Sci. Technol. 2002, 36, 163–171. [Google Scholar] [CrossRef]
- Mothé, C.G.; De Miranda, I.C. Characterization of sugarcane and coconut fibers by thermal analysis and FTIR. J. Therm. Anal. Calorim. 2009, 97, 661–665. [Google Scholar] [CrossRef]
- Castro, J.F.; Parra, C.; Yáñez-S, M.; Rojas, J.; Mendonça, R.T.; Baeza, J.; Freer, J. Optimal Pretreatment of Eucalyptus globulus by Hydrothermolysis and Alkaline Extraction for Microbial Production of Ethanol and Xylitol. Ind. Eng. Chem. Res. 2013, 52, 5713–5720. [Google Scholar] [CrossRef]
- Canevarolo, S.V., Jr. Ciência dos Polímeros. Um Texto Básico para Tecnólogos e Engenheiros; Artliber Editora: São Paulo, SP, Brazil, 2006; Volume 1, p. 277. [Google Scholar]
- Beltrami, L.V.R.; Bandeira, J.A.V.; Scienza, L.C.; Zattera, A.J. Biodegradable composites: Morphological, chemical, thermal, and mechanical properties of composites of poly(hydroxybutyrate-co-hydroxyvalerate) with curaua fibers after exposure to simulated soil. J. Appl. Polym. Sci. 2014, 131, 8769–8776. [Google Scholar] [CrossRef]
- Peng, X.; Nie, S.; Li, X.; Huang, X.; Li, Q. Characteristics of the Water- and Alkali-Soluble Hemicelluloses Fractionated by Sequential Acidification and Graded-Ethanol from Sweet Maize Stems. Molecules 2019, 24, 212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, Y.; Guo, X.; Ma, Z.; Gong, J.; Wang, H.; Lv, Y. Efficient Extraction and Structural Characterization of Hemicellulose from Sugarcane Bagasse Pith. Polymers 2020, 12, 608. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Yuan, T.; Xiao, L.; Sun, R. Effect of ultrasonic time on the structural and physico-chemical properties of hemicelluloses from Eucalyptus grandis. Carbohydr. Polym. 2018, 195, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Brinson, H.F.; Brinson, L.C. Polymer Engineering Science and Viscoelasticity: An Introduction, 2nd ed.; Springer: New York, NY, USA, 2015; ISBN 9781489974853. [Google Scholar]
- Ding, J.; Yoo, C.G.; Pu, Y.; Meng, X.; Bhagia, S.; Yu, C.; Ragauskas, A.J. Cellulolytic enzyme-aided extraction of hemicellulose from switchgrass and its characteristics. Green Chem. 2019, 21, 3902–3910. [Google Scholar] [CrossRef]
- da Silva Braga, R.; Poletto, M. Preparation and Characterization of Hemicellulose Films from Sugarcane Bagasse. Materials 2020, 13, 941. [Google Scholar] [CrossRef] [Green Version]
- Huang, B.; Tang, Y.; Pei, Q.; Zhang, K.; Liu, D.; Zhang, X. Hemicellulose-Based Films Reinforced with Unmodified and Cationically Modified Nanocrystalline Cellulose. J. Polym. Environ. 2018, 26, 1625–1634. [Google Scholar] [CrossRef]
- Zhou, C.H.; Xia, X.; Lin, C.X.; Tong, D.S.; Beltramini, J. Catalytic conversion of lignocellulosic biomass to fine chemicals and fuels. Chem. Soc. Rev. 2011, 40, 5588–5617. [Google Scholar] [CrossRef]
- Fazeli, M.; Keley, M.; Biazar, E. Preparation and characterization of starch-based composite films reinforced by cellulose nanofibers. Int. J. Biol. Macromol. 2018, 116, 272–280. [Google Scholar] [CrossRef]
- Pachekoski, W.M.; Dalmolin, C. Biodegradable Polymeric Blends of PHB and PLA for Film Production. Polím. Ciên. Tecnol. 2014, 24, 501–507. [Google Scholar] [CrossRef]
- Giaquinto, C.D.M.; De Souza, G.K.M.; Caetano, V.F.; Vinhas, G.M. Evaluation of the mechanical and thermal properties of PHB/canola oil films. Polimeros 2017, 27, 201–207. [Google Scholar] [CrossRef] [Green Version]
- Arrieta, M.P.; López, J.; Hernández, A.; Rayón, E. Ternary PLA-PHB-Limonene blends intended for biodegradable food packaging applications. Eur. Polym. J. 2014, 50, 255–270. [Google Scholar] [CrossRef]
- Jamshidian, M.; Tehrany, E.A.; Imran, M.; Akhtar, M.J.; Cleymand, F.; Desobry, S. Structural, mechanical and barrier properties of active PLA-antioxidant films. J. Food Eng. 2012, 110, 380–389. [Google Scholar] [CrossRef]
- Salam, A.; Venditti, R.A.; Pawlak, J.J.; El-Tahlawy, K. Crosslinked hemicellulose citrate-chitosan aerogel foams. Carbohydr. Polym. 2011, 84, 1221–1229. [Google Scholar] [CrossRef]
- Cassu, S.N.; Felisberti, M.I. Comportamento dinâmico-mecânico e relaxações em polímeros e blendas poliméricas. Quim. Nova 2005, 28, 255–263. [Google Scholar] [CrossRef]
- Huang, Y.F.; Kuan, W.H.; Chiueh, P.T.; Lo, S.L. Bioresource Technology A sequential method to analyze the kinetics of biomass pyrolysis. Bioresour. Technol. 2011, 102, 9241–9246. [Google Scholar] [CrossRef]






| Level | KOH Solution Concentration (% w/v) | Temperature (°C) | Time (h) |
|---|---|---|---|
| − | 10 | 25 | 3 |
| + | 20 | 50 | 5 |
| Method | Concentration (% w/v) | Temperature (°C) | Time (h) | Yield (%) | Standard Deviation | Factors | Values Effects and Interactions | t 95% * |
|---|---|---|---|---|---|---|---|---|
| 1 | 10 | ≈25 °C | 3 | 16.03 | ±0.03 | A | 1.50 | >0.24 |
| 2 | 20 | ≈25 °C | 3 | 18.11 | ±0.30 | C | 0.30 | >0.24 |
| 3 | 10 | 50 | 3 | 14.22 | ±0.14 | T | 0.02 | <0.24 |
| 4 | 20 | 50 | 3 | 15.31 | ±0.01 | t | 0.11 | <0.24 |
| 5 | 10 | ≈25 °C | 5 | 13.17 | ±0.04 | CT | −0.03 | <0.24 |
| 6 | 20 | ≈25 °C | 5 | 18.39 | ±0.22 | Ct | 0.16 | <0.24 |
| 7 | 10 | 50 | 5 | 15.97 | ±0.15 | Tt | 0.23 | <0.24 |
| 8 | 20 | 50 | 5 | 20.86 | ±0.02 | CTt | 0.02 | <0.24 |
| Method/Test Conditions | Thermal Stability (°C) | Hemicellulose | Cellulose | |||||
|---|---|---|---|---|---|---|---|---|
| Peak 1 (°C) | Peak 2 (°C) | Weight Loss at Peaks (%) | Peaks (°C) | Weight Loss (%) | Residue at 900 °C (%) | |||
| 1 | 10%_25 °C_3 h | 216 | 220 | 272 | 48 | 448 | 7 | 17 |
| 2 | 20%_25 °C_3 h | 222 | 226 | 269 | 43 | 459 | 11 | 10 |
| 3 | 10%_50 °C_3 h | 231 | 230 | 276 | 43 | 446 | 8 | 18 |
| 4 | 20%_50 °C_3 h | 233 | 229 | 274 | 46 | 455 | 10 | 11 |
| 5 | 10%_25 °C_5 h | 235 | 229 | 272 | 43 | 453 | 7 | 17 |
| 6 | 20%_25 °C_5 h | 234 | 220 | 273 | 41 | 457 | 9 | 15 |
| 7 | 10%_50 °C_5 h | 224 | 227 | 274 | 49 | 444 | 8 | 19 |
| 8 | 20%_50 °C_5 h | 220 | 225 | 272 | 42 | 455 | 11 | 10 |
| Method | H2O 3620–3500 cm−1 | CH4 3150–2740 cm−1 | CO2 2400–2240 cm−1 | CO 2230–2000 cm−1 | C=O 1850–1680 cm−1 | H2O 1500–1340 cm−1 | C-O-C 1250 cm−1 | CO2 650 cm−1 | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 10%_25 °C_3 h | 0.01 | 0.01 | 0.04 | 0.01 | 0.03 | 0.01 | 0.01 | 0.03 |
| 2 | 20%_25 °C_3 h | 0.03 | <0.01 | 0.07 | <0.01 | 0.03 | 0.01 | 0.00 | 0.03 |
| 3 | 10%_50 °C_3 h | 0.10 | 0.01 | 0.06 | 0.02 | 0.11 | 0.08 | 0.26 | 0.08 |
| 4 | 20%_50 °C_3 h | 0.03 | <0.01 | 0.15 | 0.01 | 0.03 | 0.01 | <0.01 | 0.08 |
| 5 | 10%_25 °C_5 h | 0.09 | 0.01 | 0.05 | 0.02 | 0.08 | 0.07 | 0.01 | 0.06 |
| 6 | 20%_25 °C_5 h | <0.01 | <0.01 | 0.02 | <0.01 | <0.01 | <0.01 | <0.01 | 0.01 |
| 7 | 10%_50 °C_5 h | 0.02 | 0.01 | 0.07 | 0.01 | 0.20 | 0.10 | <0.01 | 0.04 |
| 8 | 20%_50 °C_5 h | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 |
| Properties | Hemicellulose Film | Polyhydroxybutyrate Film | Polylactic Acid Film |
|---|---|---|---|
| Ultimate Tensile Strength (MPa) | 2.22 ± 0.13 | 20.87 ± 0.85 A 48.8 ± 5.3 B | 58.0 ± 2.8 C 38.5 ± 2.3 D |
| Young′s Modulus (MPa) | 4.17 ± 0.36 | 801.30 ± 24.46 A 1670 ± 50 B | 2240 ± 0.04 C 1150 ± 100 D |
| Elongation Percentage, ɛ (%) | 14.9 ± 2.65 | 4.12 ± 0.41 A 2.0 ± 0.4 B | 3.6 ± 0.2 C 1.5 ± 0.3 D |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roldi-Oliveira, M.; Diniz, L.M.; Elias, A.L.; Luz, S.M. Hemicellulose Films from Curaua Fibers (Ananas erectifolius): Extraction and Thermal and Mechanical Characterization. Polymers 2022, 14, 2999. https://doi.org/10.3390/polym14152999
Roldi-Oliveira M, Diniz LM, Elias AL, Luz SM. Hemicellulose Films from Curaua Fibers (Ananas erectifolius): Extraction and Thermal and Mechanical Characterization. Polymers. 2022; 14(15):2999. https://doi.org/10.3390/polym14152999
Chicago/Turabian StyleRoldi-Oliveira, Mariana, Layse M. Diniz, Anastasia L. Elias, and Sandra M. Luz. 2022. "Hemicellulose Films from Curaua Fibers (Ananas erectifolius): Extraction and Thermal and Mechanical Characterization" Polymers 14, no. 15: 2999. https://doi.org/10.3390/polym14152999